Showing 6253 Results
SEARCH
Webinars
(17)
Virtual Events
(15)
Trending News
(768)
Content Tags
(3)
Users
(5423)
Scientific Products
(27)
-
This series will explore historic space missions from the start of the Space Age to the present day, including both crewed and robotic missions. Here we wi
FEB 15, 2017
Space & Astronomy
The International Space Station is used every day by astronauts from official space agencies of countries around the world, including, but not limited to,
SEP 10, 2015
Cancer
A drug-like molecule could prove valuable in cancer research with the potential of developing a new pharmaceutical. Researchers from the Ontario Instit
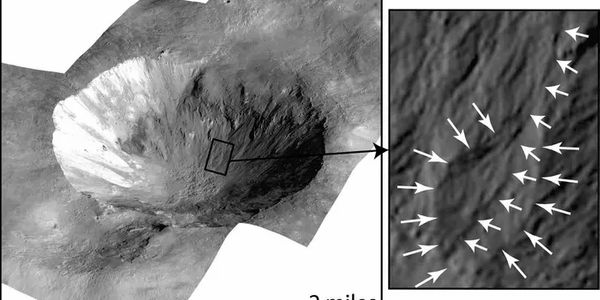
Following a two-year journey through our solar system, NASA’s Origins, Spectral Interpretation, Resource Identification, Security-Regolith Explorer (
JAN 28, 2019
Space & Astronomy
It’s a big year for commercial space companies like Boeing and SpaceX; both have worked extraordinarily hard to bring crewed rocket launches back to
MAR 03, 2019
Space & Astronomy
Just as expected, SpaceX moved forward with a momentous end-to-end demonstration launch for NASA on Saturday, verifying once and for all that the Falcon 9
AUG 07, 2016
Clinical & Molecular DX
When we need medicine, we hop down to the local drugstore or pharmacy and have our prescriptions filled. But what happens when doctors or emergency respond
AUG 27, 2022
Space & Astronomy
This series will explore historic space missions from the start of the Space Age to the present day, including both crewed and robotic missions. Here we wi
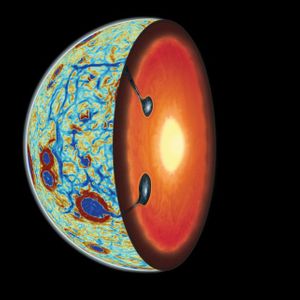
In May 2018, NASA announced the three teams that would be receiving five-year grants of about $8 million each to study astrobiology: the origins, evolution