A cutting-edge therapy that uses a person’s own immune cells to kill cancer just received a historic vote of confidence from the US Food and Drug advisory committee. Indeed, after reviewing promising clinical trial data from Novartis, the panel recommended that CAR T-cell immunotherapy be approved for a type of leukemia, known as B-cell acute lymphoblastic leukemia (ALL).
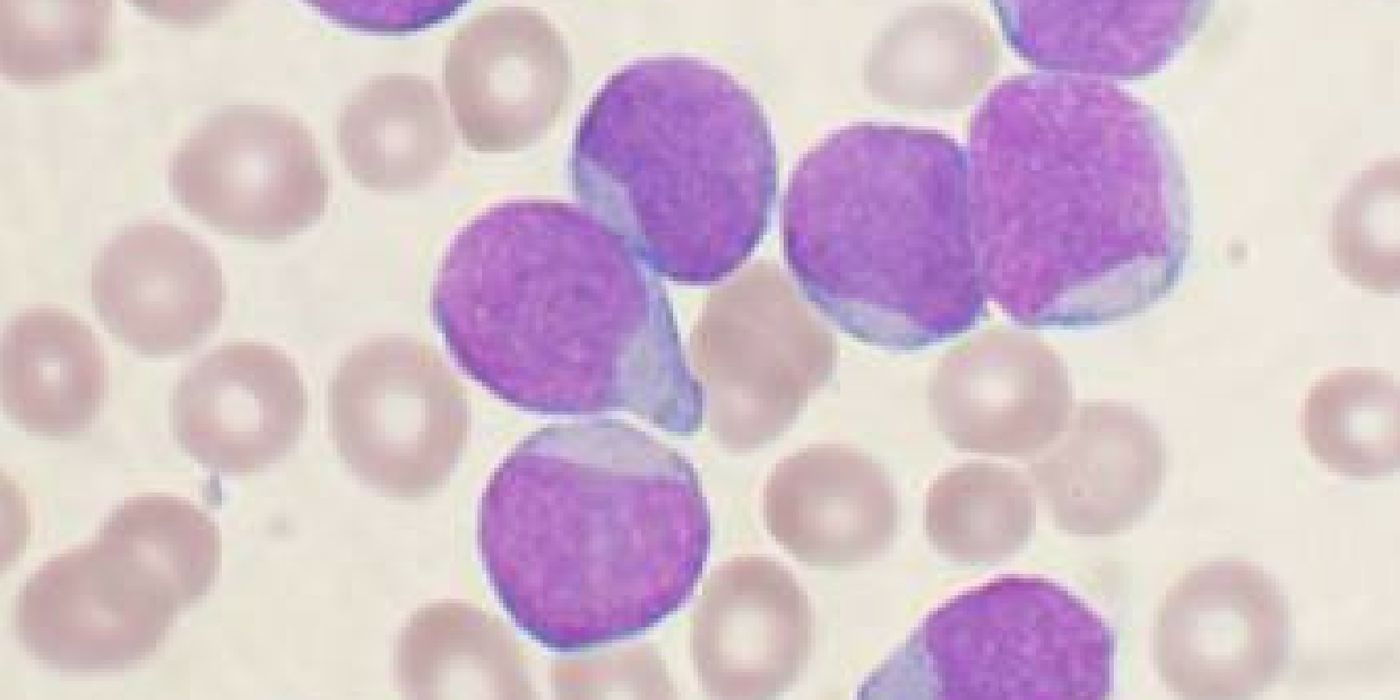
Though considered a rare disease, acute lymphoblastic leukemia (ALL) is the most common form of childhood cancer, peaking in early childhood between the ages of 2 and 4. The cancer involves the overproduction of immature white blood cells in the bone marrow. These cancerous cells invade the blood and other organ systems, causing massive cell death and damage in a short amount of time. Radiation therapy, bone marrow transplantation, and chemotherapy agents like methotrexate, vincristine, and cytarabine, are effective against ALL. However, it doesn’t work for everyone, and remission isn’t a sustained outcome in 15 to 20 percent of children in whom the cancer makes a dreaded comeback.
Immunotherapy is a type of cancer treatment that stimulates the body’s natural defenses to kill cancer cells. The form of immunotherapy tested in the latest Phase 2 clinical trial involves modifying the patient’s immune T-cells and genetically alter them to carry a synthetic receptor molecule called a CAR (for chimeric antigen receptor). These specialized CAR T-cells are then able to recognize and destroy cancer cells that have a specific marker, known as CD19.
Unlike a conventional cancer drug, the CAR T-cell therapy is more potent because it can be self-sustaining. That is, even though it’s a one-time therapy, once re-introduced into the patient’s body, the engineered T-cells are expected to multiple and form an army against the cancer cells. This means that patients should be continually protected by the ‘living drug,’ much in the same way that vaccines protect us against pathogens.
In the Phase 2 trial, Novartis treated 63 pediatric patients with ALL who had exhausted all other approved treatment options. Of the treated patients, 83 percent (52 children) went into remission. The other 11 children had passed away.
Based on these results, the panel passed a unanimous vote 10-0 in favor of approving the treatment.
"I think this is most exciting thing I’ve seen in my lifetime," said Dr. Tim Cripe, an oncologist who was on the panel. While the panel’s recommendation isn’t the final say, their nod will be a factor in the FDA’s final ruling come October 2017.
"The panel's unanimous recommendation in favor of CTL019 moves us closer to potentially delivering the first-ever commercially approved CAR-T cell therapy to patients in need," Novartis Oncology CEO Bruno Strigini said in a news release.
As life-changing as this therapy can be, it’s not without dramatic risks. When the reengineered T-cells start hunting and killing cancer cells, it can trigger cytokine release syndrome, which causes fever and neurotoxicities in the patients. Patients have to be under intense surveillance during this process, which can take weeks. Of note, patient deaths have been associated with the therapy by competing pharmaceutical companies (Kite Pharma and Juno Therapeutics).
The CAR T-cell immunotherapy also comes with a hefty price tag of at least $300,000. And it’ll only be available for patients who have already exhausted (and footed the bill for) other treatment options.
Of note, the long-term effects of this therapy is still under investigation. Patients in this trial will be monitored for a total of 15 years to answer this question.
Additional sources: Novartis, Live Science