Engineering a Better Heart Valve
Over 5 million Americans have heart valve disease (HVD), which can lead to heart failure, stroke, blood clots, and even death. HVD occurs when one or more of the four valves of the heart does not work properly, disrupting the flow of blood through your heart.
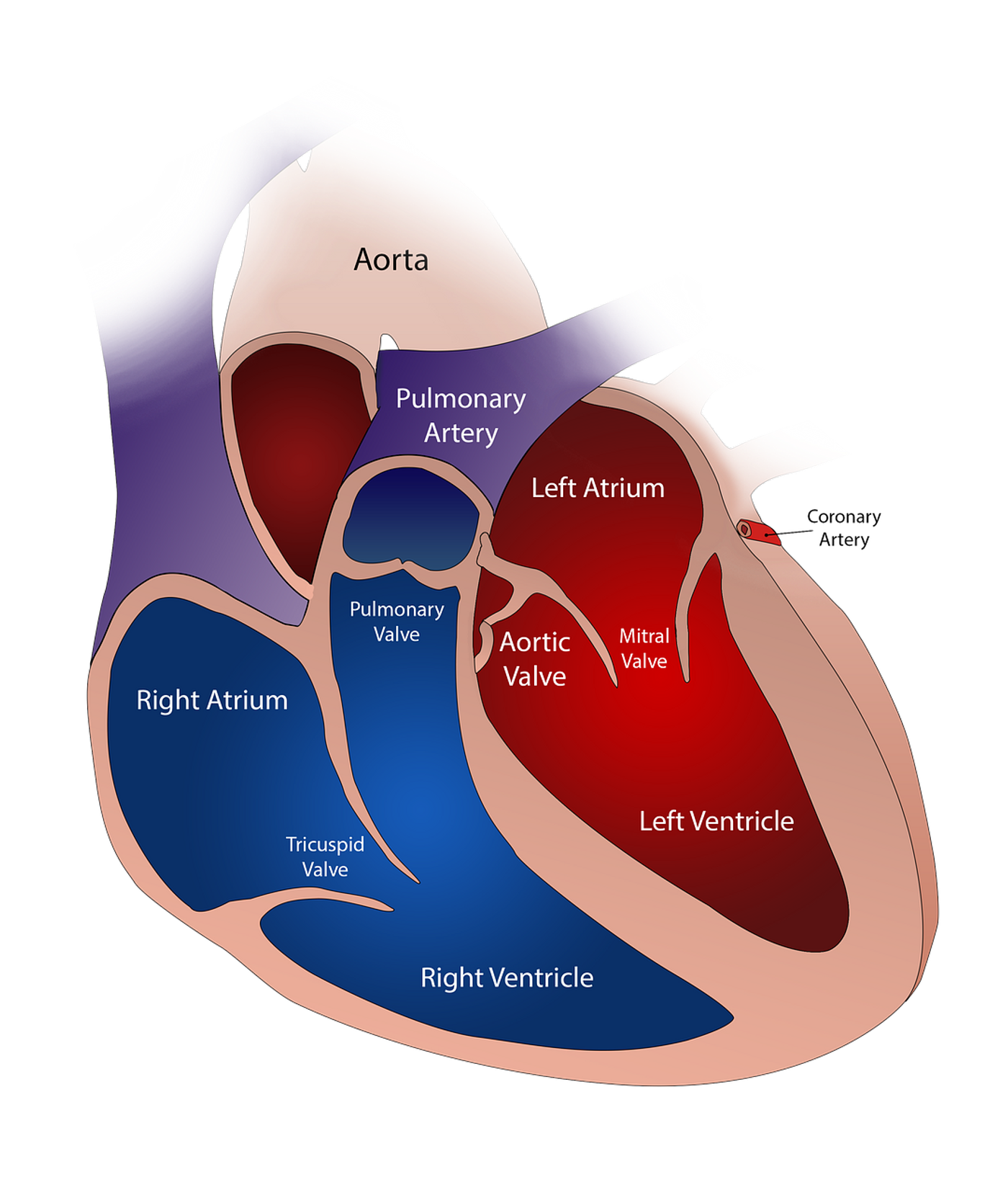
The human heart has four valves: aortic, pulmonary, mitral and tricuspid. When blood is pumped through the heart it passes in one direction, like a one-way street, through these valves to the four chambers of the heart. The valves open to allow blood to flow through, and close to prevent the backwards flow of blood. In HVD a valve may be too narrow preventing proper blood flow or not open/close properly creating a “leak”.
In some cases, treatment of HVD can involve the replacement of the malfunctioning valve. Current options for replacement can include mechanical and tissue valves made of human or animal donor tissue. Use of mechanical valves does not allow for the prostheses to grow or adapt to change with a human body. This is particularly problematic for younger patients who are still growing. Alternatively, tissue-engineered heart valves have been tested but have so far failed. Implementation of tissue-engineered heart valves leads to detrimental valve remodeling that impairs the normal function of the valves.
Previous work has been done to use tissue engineering to grow tissues in the shape of a valve, creating an extracellular-matrix scaffold that is implanted in the heart. The scaffold can then be populated by the human recipients own cells, with some transforming in to contractile cells, to create new tissue and remodel the valve. As mentioned above, animal testing has shown problems such as excessive tissue production, with use of a tissue-engineered valve. The excessive production of tissue, called fibrosis, can be caused by biomechanical stresses that lead to leaflet thickening or shortening and retraction. Which in turn leads to improper blood flow from narrowing of the valve or creates a “leaky” valve that does not properly open/close.
A recent study from the University of Zurich has turned to computational modeling and bioengineering to find a solution to this problem. The group sought an alternative approach that would prevent excessive activation of contractive-cells during the remodeling process. By doing so they could attempt to limit leaflet shortening and retraction that occurred in previously designed valves. To test this approach, they created computationally designed tissue-engineered heart valves to replace valves in sheep. Over the course of a year they assessed the performance of the valves and found that they adopted a stable valve shape as the model had predicted. While these valves did initially shorten during remodeling they stabilized after six months allowing for proper valve function comparable to native valves in 90% of the sheep.
While this study has shown the ability of predictive-modelling to successfully create a tissue-engineered heart valve that prevents the issue of fibrosis, the study utilized only healthy sheep. In a healthy sheep contractile cell activation is low, while in humans this may differ when dealing with individuals suffering from valve disease. In this case contractile-cell activation, driven by the immune response, may be high which could lead to fibrosis and failure of the computationally modeled tissue-engineered valve. Further studies will need to be completed to determine the robustness and longevity of this strategy past a 12-month time period and in various conditions. But this study has demonstrated promising potential for utilizing computational methods to create tissue-engineered heart valves based on predicted outcomes.
Click here to read the study. To learn more about the function of heart valves and what problems can arise see the video below.