Dogs and humans alike can suffer from a rare bleeding disorder called factor VII deficiency, where lacking a vital coagulation protein, factor VII, can lead to extensive bleeding in the central nervous system or the gastrointestinal track, often after surgery or an injury. New success in gene therapy techniques have been successful in treating dogs with factor VII deficiency, and scientists are hopeful that the therapy will work equally as well in humans.
Scientists from the Children’s Hospital of Philadelphia (CHOP) in collaboration with a team from the University of Chapel Hill have bioengineered an adeno-associated virus to work as a non-infectious vector for delivering DNA into deficient cells. This DNA carries the information to produce clotting proteins to reverse the effect of the disorder. Their study was published this week in the journal
Blood.
Factor VII deficiency has historically been treated with infusions of clotting factor to make up for the missing proteins, but after scientists saw success with gene therapies for hemophilia B, studies began to use a similar technique to solve this disorder.
Scientists found that the same mutation causing factor VII deficiency in dogs also occurs in most human cases of the disease. After treating dogs with the deficiency with a single injection of the corrective DNA vector, the scientists involved in this study saw the dogs expressing “levels of clotting factor VII that would be therapeutic in humans with long-term stability.”
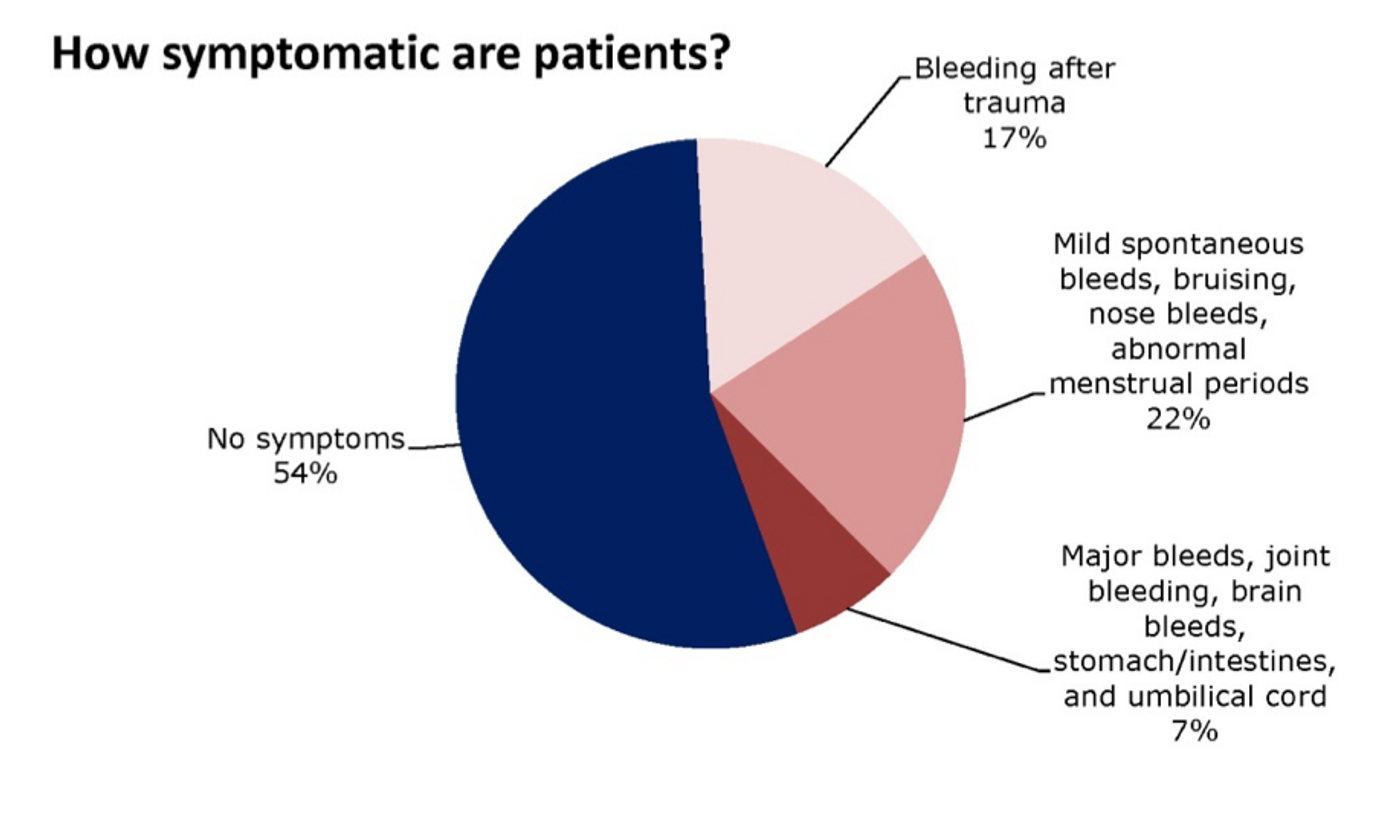
For the one in 300,000 to 500,000 people who have factor VII deficiency, the promise of a new therapy to improve blood coagulation could mean a drastic increase in their quality of life. The severity of the deficiency varies in different cases, with 40 percent of patients experiencing the most severe side of the spectrum. Males and females are equally likely to have this disorder, but females with the mutation suffer excessive menstrual bleeding in addition to the post-surgery or post-injury repercussions.
"The FVII-deficient dogs tolerated the initial gene therapy infusions very well and have had no adverse side effects over several years of follow up,” said Tim Nichols, MD, who led the group of scientists from UNC. Lastly, the gene therapy trials in the dog models of disease were assured to be a safe treatment without “unwanted immune responses.”
Source:
Children's Hospital of Philadelphia