Rare Aging Disease Thwarted By New Technology
Most other current research on progeria, a rare and fatal genetic aging condition, revolves around genetics, but John P. Cooke, MD, PhD, from the Houston Methodist Research Institute, is looking at telomeres. As a new approach for age reversal at a cellular level, Cooke is aiming to extend telomeres that sometimes shrink, causing accelerated aging.
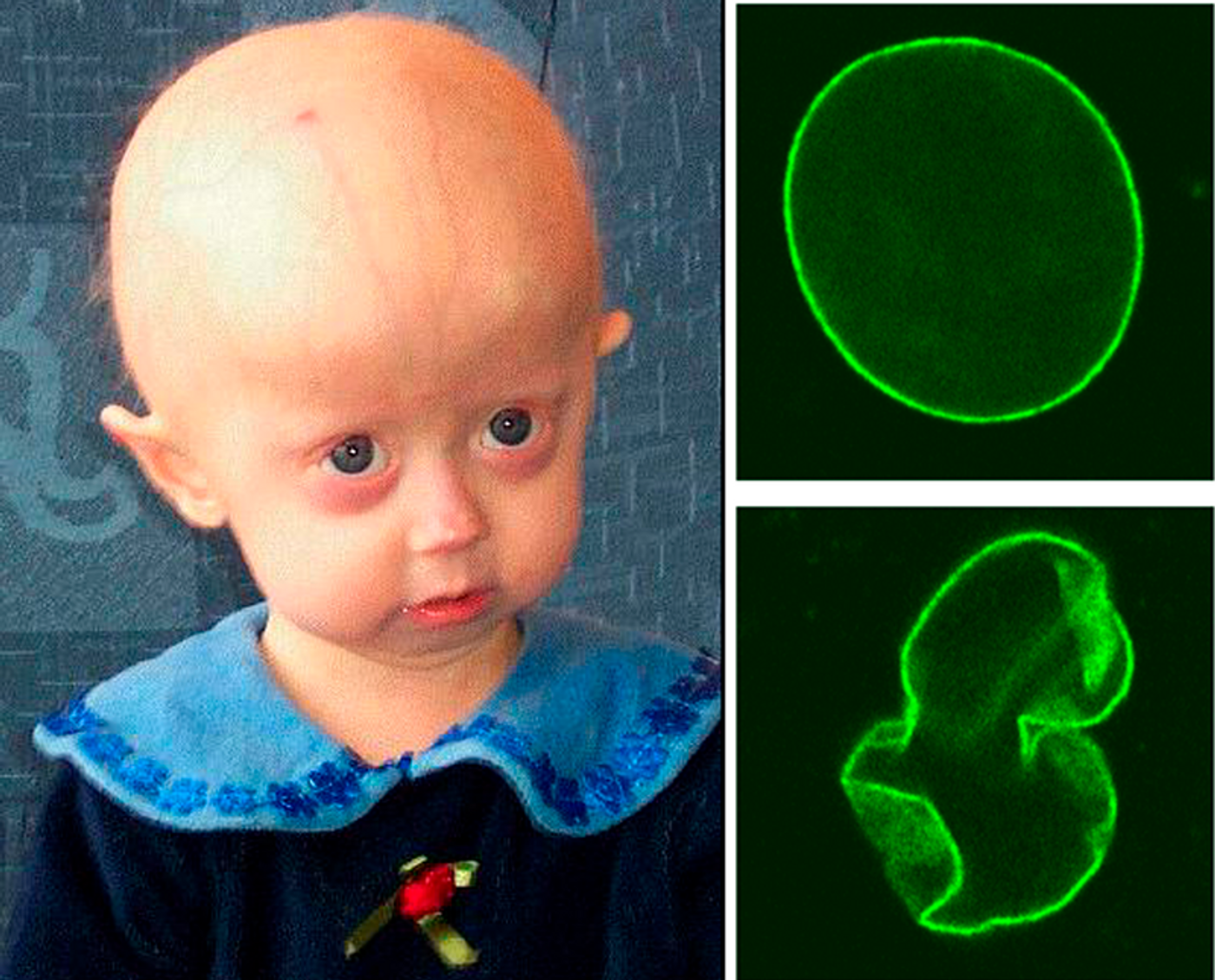
Hutchinson-Gilford Progeria Syndrome (Progeria, HGPS) is caused by a mutation in a gene called LMNA, which inhibits the production of a protein necessary for stabilizing a cell’s nucleus. An unstable nucleus ultimately results in accelerated aging that usually becomes visible by the time a child reaches two years old; children with progeria usually die by fourteen years old of atherosclerosis.
“We wanted to do something that would improve the children's quality of life and potentially allow them to live longer, so we set about studying their cells and seeing if we could improve the cell function,” Cooke explained.
Telomeres are sequences of nucleotides that cap the ends of chromosomes, heavily intertwined in their function. As we get older, our telomeres get shorter. And in children with progeria, their telomeres shrink at an alarming rate, Cooke found. Would bringing telomere length back to a normal level reverse the effects of the disease?
"We all have telomere erosion over time, and many of the things that happen to these children at an accelerated pace occur in all of us," Cooke explained. "What we've shown is that when we reverse the process of the telomere shortening in the cells from these children and lengthen them, it can reverse a lot of the problems associated with aging."
In attempt to apply his theory of telomere restoration, Cooke implemented something called RNA therapeutics. By delivering telomerase-encoding RNA, Cooke could induce its production; telomerase is an enzyme that can elongate telomeres. After RNA therapeutics, Cooke was somewhat surprised by the result.
"We were not expecting to see such a dramatic effect on the ability of the cells to proliferate,” Cooke explained. “They could function and divide more normally, and we gave them extra lifespan, as well as better function."
Cooke says aging is a major risk factor for heart disease in general, not just for children with progeria. "About a third of the people in this country succumb to strokes and heart attacks,” he said. “If we can fix that, we'll fix a lot of diseases."
Aging is not completely irreversible, and Cooke’s work isn’t for immortality. "We can at least stall or slow down accelerated aging, and that's what we're working toward," Cooke concluded. "Our next steps are to start moving this therapy toward clinical use. We plan to do so by improving existing cell therapies. I want to develop a therapy for these children. It's an unmet need."
The present study was published in the Journal of the American Heart Association.