Single Cells to Thank for Rebuilding Blood Vessels
During a process called angiogenesis, new blood vessels form as cells divide continuously to create new layers of tissue. But is the division of those cells random, or are specific cells called into action to ensure new blood vessels form after tissue injury from a heart attack or during development to build healthy vasculature? Scientists from Goethe University Frankfurt tackled this question in their most recent study.
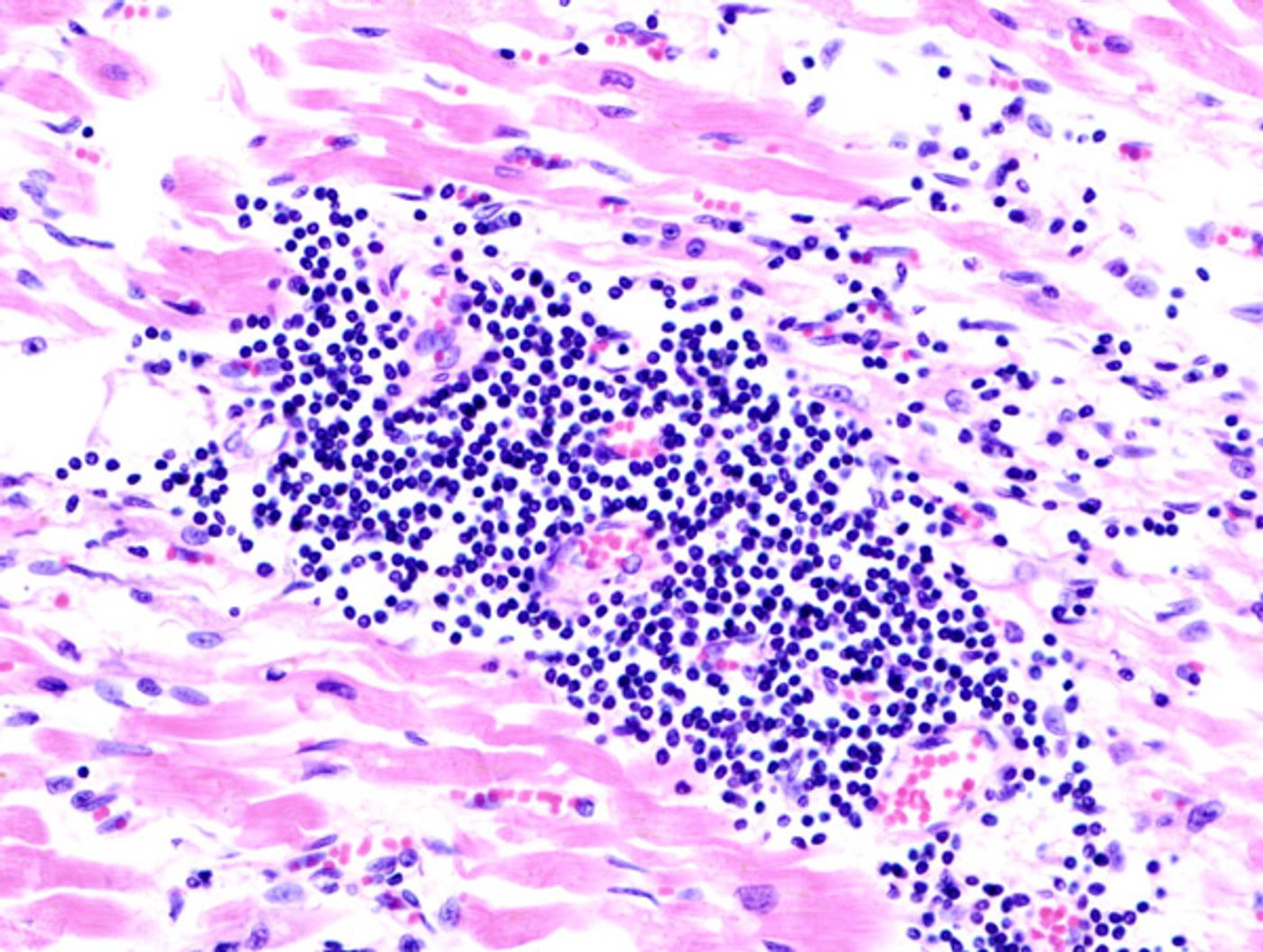
Researchers used an animal model called Confetti mice to study single cells called endothelial cells, which make up the innermost layer, the endothelium, of blood vessels. From past findings, researchers knew that endothelial cells were to thank for multiplying after injury to promote angiogenesis and recovery, but little details surrounding this activity were available.
Confetti mice are animal models used by scientists to tag specific cell types within tissue with fluorescent proteins. When these proteins fluoresce under the right laboratory conditions, researchers can distinguish cells of interest from the other cells of the body. In the present study, researchers designed their animal models so that only endothelial cells would fluoresce, and in three different colors.
Additionally, when each fluorescently-tagged cell divides, the result of that division and subsequent divisions continue to fluoresce, allowing researchers to track many generations of endothelial cells.
Their first observation was that angiogenesis post-injury from heart attack consisted of certain cells from damaged heart tissue dividing repeatedly. Additionally, researchers saw the same specific division in heart attack-damaged skeletal muscle tissue.
"It might be that clonal expansion is no longer that efficient in older people, which is why a lot of damaged tissue dies off after a heart attack and forms scar tissue which cannot be reactivated through the formation of new blood vessels," said Frankfurt Professor Stefanie Dimmeler. "If we manage to characterize the clonally expanding cells more precisely, we will hopefully find ways to re-stimulate this process."
Dimmeler also observed a “surprising” ratio of clonally expanding cells: 30 to 50 percent.
“Perhaps we're even underestimating the ratio of clonal expansion," Dimmeler said. "Because after all we haven't conducted a three-dimensional analysis but instead identified the fluorescing cells in two-dimensional tissue slices."
Their second observation was that unlike in damaged heart tissue, newborn models showed no clonal expansion in retinal angiogenesis. Blood vessel growth in this context was instead assumed to be a result of random cell division.
Going forward, researchers want to get a clearer view of the “fate” of endothelial cells long-term.
"We want to know what has happened to these cells after a year and whether the new blood vessels are just as good as the old ones in the long term," Dimmeler explained.
Further additions to this growing field of knowledge could have a huge impact on treating tissue damage for people with diabetes or patients recovering from heart attack.
The present study was published in the journal Circulation Research.