Scientists have used model organisms to view the first few hours of development in various organisms. A single cell is fertilized, and cell division immediately begins, proliferating into a mass that will eventually form an embryo. Cells are organizing during this time, moving into position and generating the structures that an animal will need. Researchers have now identified an important regulator of the process that helps cells get organized. The findings have been reported in Science.
Using zebrafish to study how the spinal cord forms, scientists identified unique combinations of molecules that cells use to get properly sorted, so-called adhesion codes that can determine the cells that stay linked, and how strongly they stay together as cells are rearranged in the growing animal. Master signaling molecules called morphogens can control adhesion codes.
"My lab's goal is to understand the basic design principles of biological form," said the co-corresponding study author Sean Megason, professor of systems biology in the Blavatnik Institute at Harvard Medical School. "Our findings represent a new way of approaching the question of morphogenesis, which is one of the oldest and most important in embryology. We see this as the tip of the iceberg for such efforts."
This work may aid in the creation of engineered tissue that might be useful in transplantation, for example.
"Constructing artificial tissues for research or medical applications is a critically important goal, but currently one of the biggest problems is inconsistency," said lead study author Tony Tsai, a research fellow in systems biology in the Blavatnik Institute. "There is a clear lesson to learn from understanding and reverse engineering how cells in a developing embryo are able to build the components of an organism in such a robust and reproducible way."
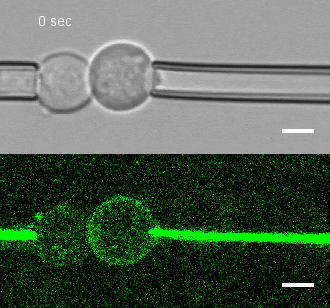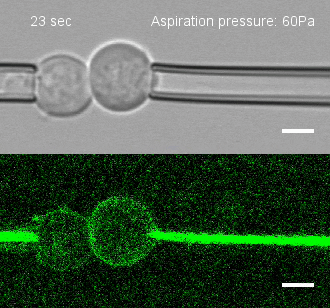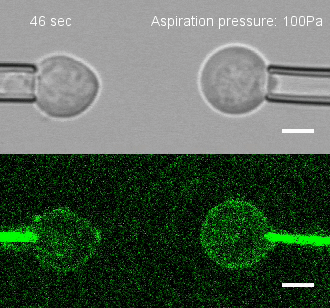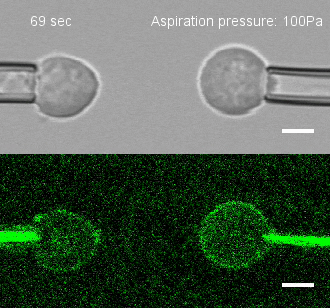
The researchers developed a technique for measuring the adhesion force or establishing adhesion preferences between cells. Individual cells could be put together and then precisely pulled apart by micropipettes. This method was used to investigate the behavior of cells that give rise to the spinal cord, or neural progenitor cells, in zebrafish embryos.
Similar cells were found to strongly prefer one another. The scientists applied gene expression analysis tools to find the genes that are involved in producing adhesion molecules. After identifying the genes, they were able to use CRISPR-Cas9 to block one gene at a time. Then they applied the pulling assay to any that caused disruption.
Three genes were found to be essential for generating normal patterns: N-cadherin, cadherin 11, and protocadherin 19. The expression levels of the genes acted as a kind of code that could alter adhesion preferences.
"All three adhesion molecules we looked at are expressed in different amounts in each cell type," Tsai said. "Cells use this code to preferentially adhere to cells of their own type, which is what allows different cell types to separate during pattern formation. But cells also maintain some level of adhesion with other cell types since they have to collaborate to form tissues. By piecing together these local interaction rules, we can illuminate the global picture."
Only three types of cells were analyzed in this study, so the researchers want to look at other types of cells now. They also want to know more about the effect of morphogens on adhesion. If we can learn more about how one cell can grow to be a whole organism, we may be able to create artificial organs.
"The issue with tissue engineering right now is that we just don't know what the underlying science is," Megason said. "If you want to build a little bridge over a stream, maybe you could do that without understanding physics. But if you wanted to build a big suspension bridge, you need to know a lot about the underlying physics. Our goal is to figure out what those rules are for the embryo."
Sources: AAAS/Eurekalert! Via Harvard Medical School, Science