How SARS-CoV-2 Evades Antiviral Defenses
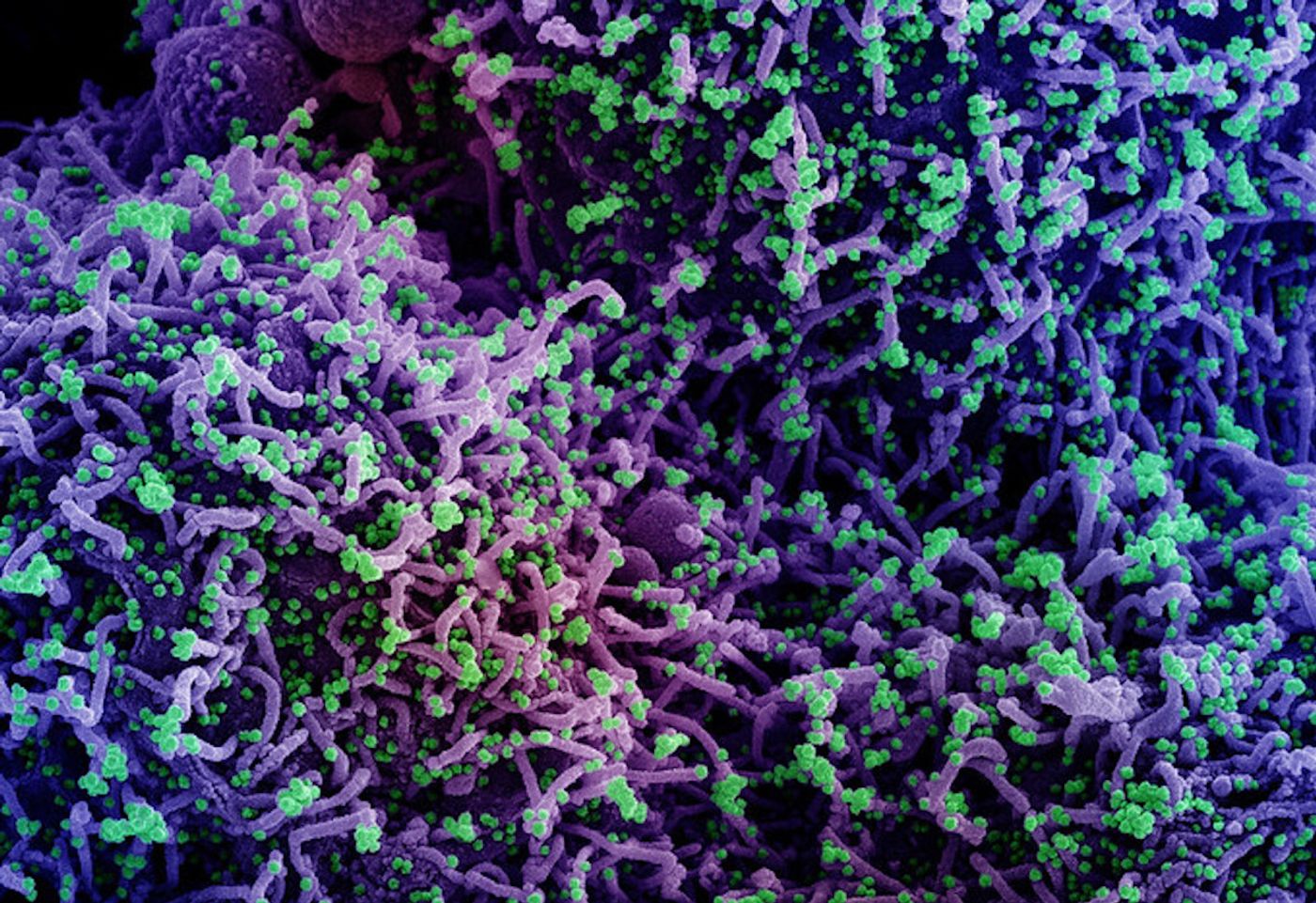
Researchers have learned a lot about the SARS-CoV-2 virus since the start of the COVID-19 pandemic. We know that the virus has a spike protein that enables it to latch on to epithelial cells in the nose, and gain entry to those cells to cause an infection. Once that infection takes hold, it can cause serious disease and infect more cells in the respiratory system. Scientists have now learned more about how the SARS-CoV-2 virus prevents immune cells from detecting infected cells, which allows the virus to persist and spread through the body. The findings have been published in Cell Reports.
In this study, the researchers discovered that a protein on human host cells called galectin-8, which normally acts as a sensor and can help defend against infection, binds to the SARS-CoV-2 spike protein. The virus takes that defense mechanism out of commission with a viral enzyme called 3CL protease, which targets galactic-8 and cuts it in half.
"We discovered that the virus attaches to and deactivates an important sensor protein in the host cell called galectin-8, which protects the cell against infection. By deactivating galectin-8, SARS-CoV-2 disarms a cell's antiviral defense system and allows the virus to take over the host," explained senior study author Dr. Chris Overall of the UBC Centre for Blood Research, Life Sciences Institute and Faculty of Dentistry.
Normally, galectin-8 is involved in a process called xenophagy, in which stuff like viruses that invade cells get rounded up in small sacs and injected with destructive molecules.
First study author Isabel Pablos, noted that the viral enzyme can impair galactic-8 in a way that prevents it from destroying the virus. The 3CL protease, like the spike protein, is a feature of other coronaviruses. But in the case of SARS-CoV-2, the 3CL protease seems to have more than one function that promotes viral infection.
The study authors analyzed lung tissue from COVID-19 patients, and found that galactic-8 had been disrupted in infected lung cells.
The research also identified about 150 host cell proteins that can be targeted and deactivated by the viral protease. This may be a key reason why the virus is so successful at causing infection in some patients. The protease seems to be able to target the Hippo pathway in particular; four enzyme cut sites were found there, suggesting that the virus can impair the normal functions of cells and encourage viral replication.
"By understanding how SARS-CoV-2 hinders a host cell's ability to defend itself, and identifying which molecular sites are cut in order to accomplish this, we can finally understand how the virus hijacks the cell," says Overall.
This research may be particularly useful to scientists that are trying to develop treatments for COVID-19.
Sources: The University of British Columbia (UBC), Cell Reports