Neurodegenerative Disease Development is Linked to the Largest Organelle
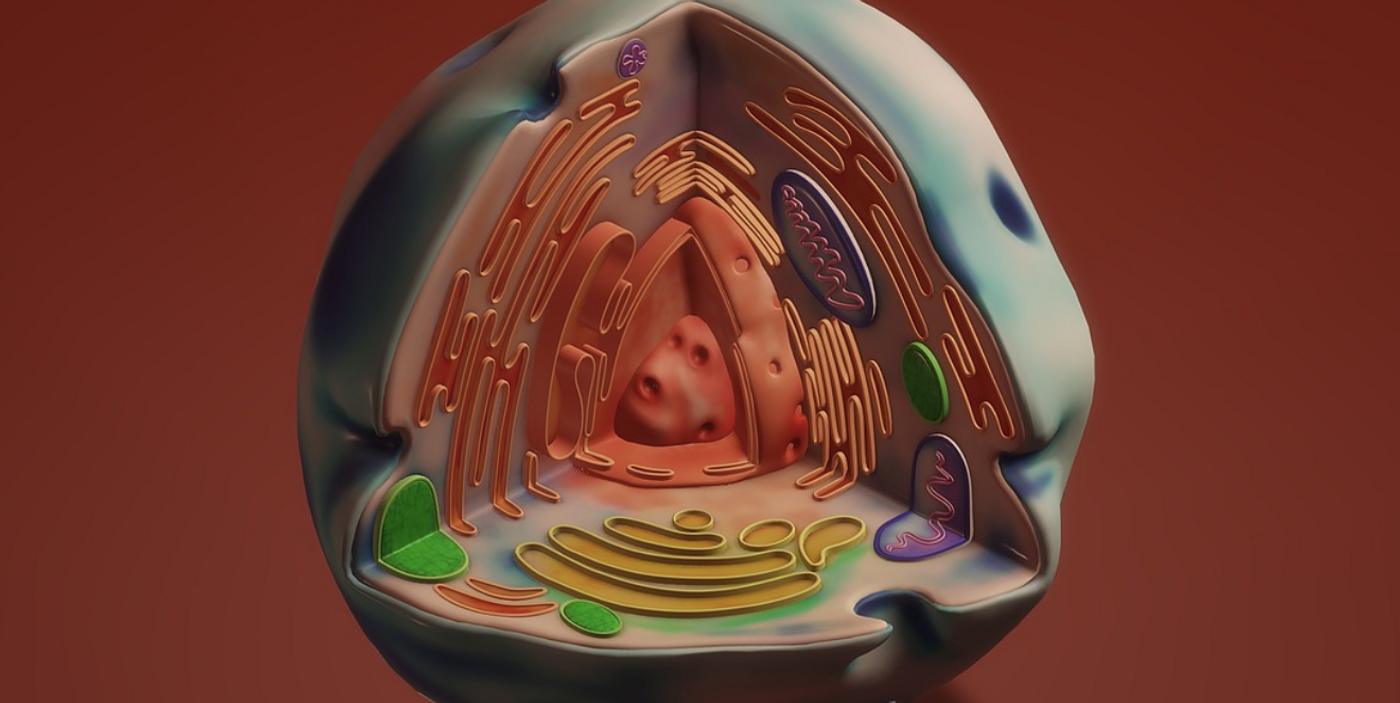
The endoplasmic reticulum (ER) is the single biggest structure in eukaryotic cells, and it's made up of various shapes, like sheets and tubules, which are interconnected. The ER is critical to many processes, including cell signaling, protein production and degradation, and fat distribution. In the ER, proteins are generated, properly folded into a three dimensional structure, and modified in various ways. The quality of the ER is under constantly monitoring, and certain parts cen be selectively degraded; this process is called ER-phagy. The ER can also be built back up again. Two new papers reported in Nature have revealed more about how this renewal and degradation process is linked to a crucial molecule called ubiquitin, and dysfunctions in these processes are associated with neurodegeneration.
Ubiquitin is a tag that can be added to proteins after they have been generated - it is a post-translational modifcation that plays a role in many cellular functions, including the disposal of proteins that are misfolded or dysfunctional, and ER-phagy.
Various proteins and receptors help control the form of the ER. In ER-phagy, receptors build up at certain sites, and increase the physical curvature of the ER, cutting off portions that are broken down into components by structures called autophagosomes.
Ubiquitin helps a protein called FAM134B, a membrane curvature receptor, cluster in the ER membrane. Ubiquitin was thus found to drive ER-phagy. Researchers have also shown that when the FAM134B protein is dysfunctional, ER-phagy is as well, and neurodegeneration can occur.
Mutations in FAM134B can cause a rare disorder in which sensory nerves die, and patients can't sense temperature or pain. Patients may not notice wounds or injuries, which can become chronic.
FAM134B clusters are more stable because of ubiquitin and the ER bulges out where these clusters appear. Additional curvature in the membrane further stabilizes the clusters, which attracts even more membrane curvature proteins. Thus, ubiquitin has a self-reinforcing effect, said Professor Ivan Đikić of Goethe University Frankfurt. Ubiquitin can also alter part of the FAM134B protein's shape. "This is another facet of ubiquitin that performs an almost unbelievable array of tasks to keep all different cell functions working,” added Đikić.
When mutations arise in ARL6IP1, another membrane curvature protein, it can cause a neurodegenerative disease that manifests as hardening in leg muscles and sensory defects. ARL6IP1 was found to play a role in ER-phagy. A ubiquitin tag is also added to this protein, meaning it is ubiquitinated during ER-phagy.
When mice lack ARL6IP1, the ER gets bigger and degenerates as cells age, leading to the accumulation of misfolded proteins or clumps of proteins. Cells stop disposing of these clumps, which is especially problematic for nerve cells that don't renew that often, causing the cells to die. This leads to patient symptoms, explained Professor Christian Hübner of Jena University Hospital.
The membrane curvature receptors FAM134B and ARL6IP1 seem to form mixed clusters during ER-phagy, altering the size and function of ER.
“We now understand better how cells control their functions and thus create something we call cellular homeostasis. In biology, this knowledge allows fascinating insights into the incredible achievements of our cells, and for medicine it is essential for understanding diseases, diagnosing them on time and helping patients by developing new therapies," said Đikić.
Sources: Goethe University Frankfurt am Main, González et al Nature 2023, Foronda et al Nature 2023