The amyloid-β peptide (Aβ) is a crucial protein in the development of Alzheimer’s disease (AD). Thought to cause the death of neurons, Aβ can aggregate to form insoluble aggregates, known as amyloid plaques, in the brains of patients with AD. They are one of the hallmarks of the disease.
It’s been
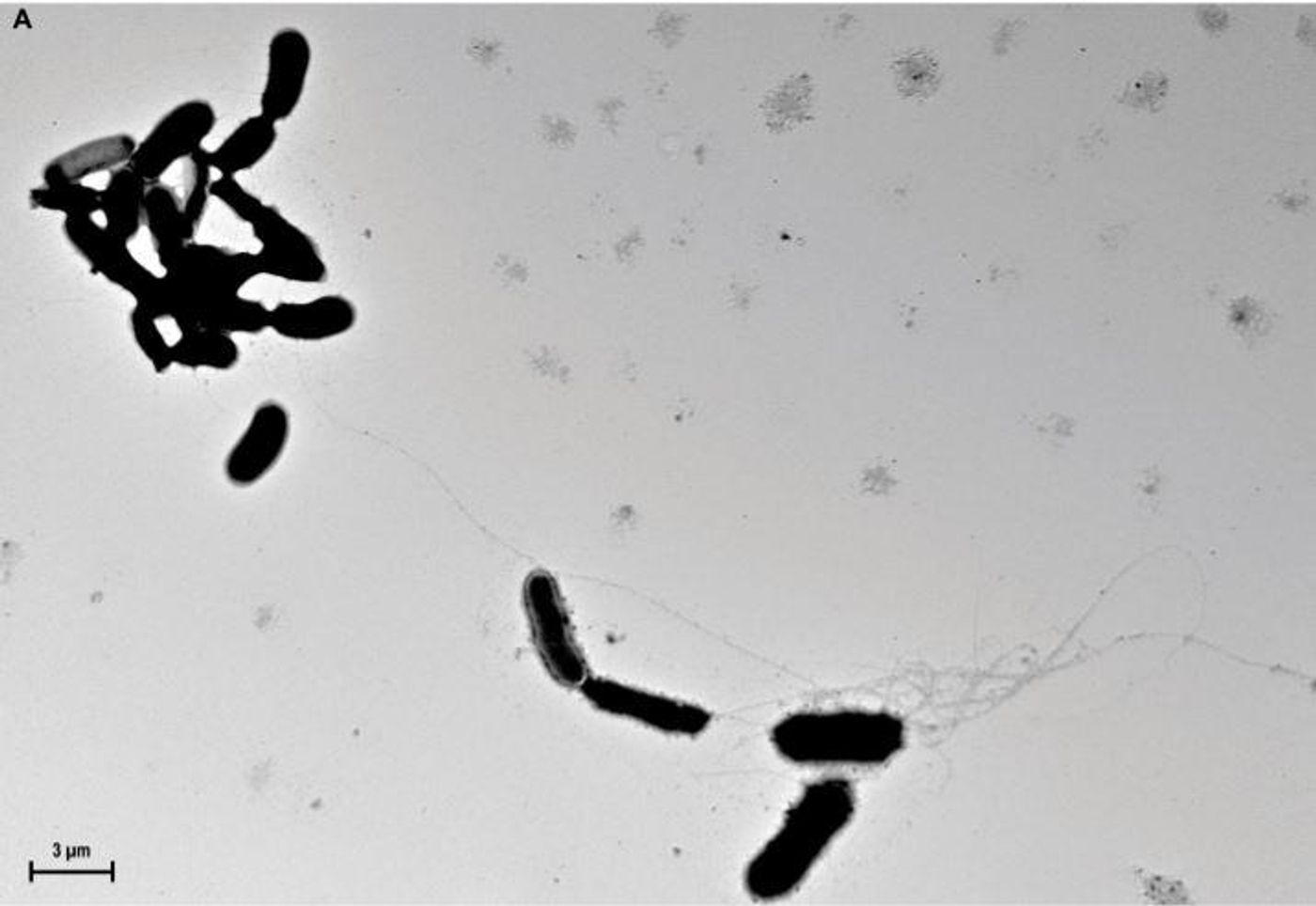
previously reported that Aβ is an antimicrobial peptide. One characteristic of Aβ peptides is oligomerization, or the formation of supramolecular structures. Usually seen as inherently pathological,
a new report by Robert D. Moir, Rudolph E. Tanzi, and colleagues at Harvard Medical School, in Science Translational Medicine suggests that oligomerization may actually be necessary for the antimicrobial action of the peptide; that it allows Aβ to form a structure that captures the infecting organism. The work also shows that the expression of Aβ guards against bacterial and fungal infections in cell culture, nematode, and mouse models of AD.
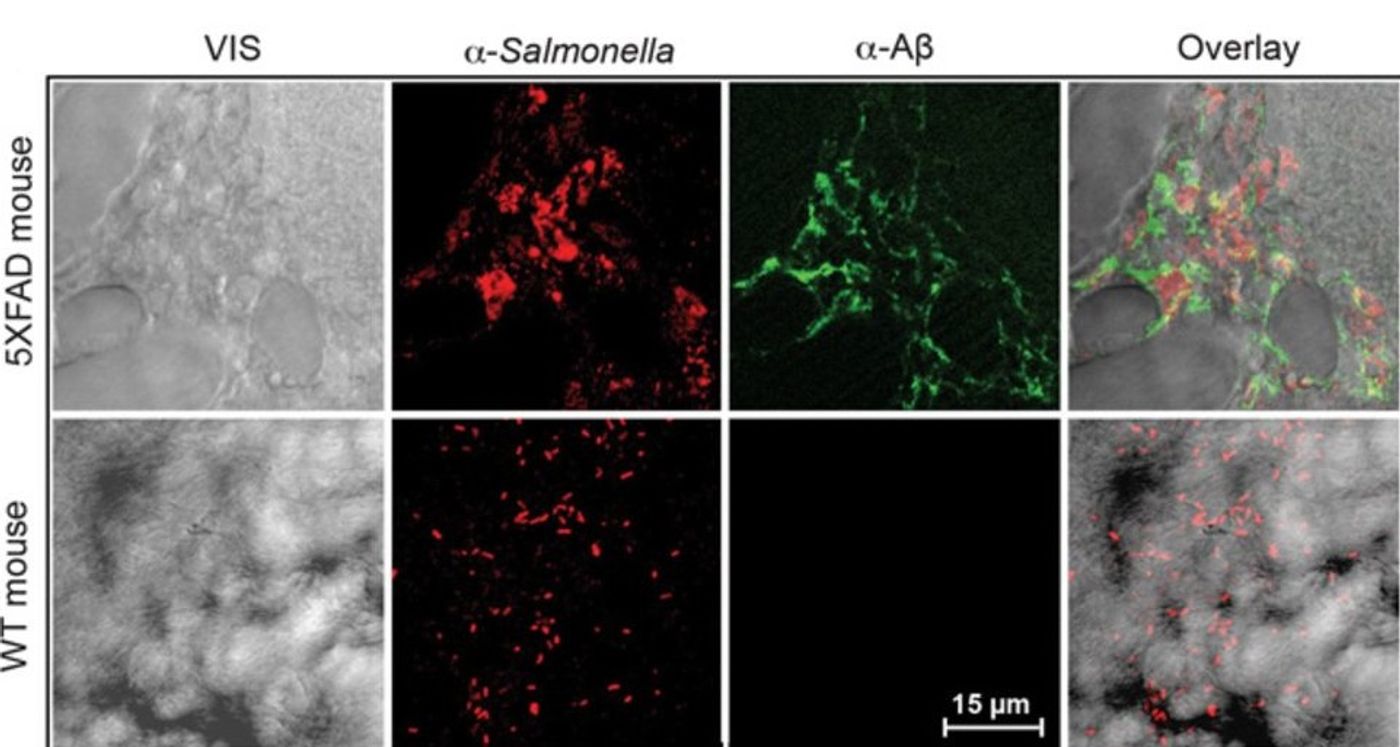
As part of the study, researchers injected Salmonella into young mouse brains that were free of plaques. They observed a rapid accumulation of β-amyloid deposits, and those buildups were closely aligned with the location of a bacterial infection. “Overnight, the bacteria seeded plaques,” Dr. Tanzi said. “The hippocampus was full of plaques, and each plaque had a single bacterium at its center.”
To verify the antibacterial properties of Aβ, researchers checked mice that don’t produce any Aβ. It was found that they are not able to fight off bacterial infections. They have confirmed that result in nematodes and cell culture. Mice lacking Aβ also do not form plaques.
The work builds on the idea that as the blood-brain barrier gets weak with age, it can’t keep infections out. Aβ comes in to do its work as an antimicrobial agent, but after the invader is killed, what’s left behind damages the brain.
This new research is very exciting for the field. It might be the connection between disparate findings, such as high levels of a herpes antibody in some AD patients. The director of the Zilkha Neurogenetic Institute at the University of Southern California, Dr. Berislav Zlokovic, said his research on the blood-brain barrier would fit well with this new hypothesis. He discovered that as people age, the barrier started to break down, and the most damaged part was around the hippocampus, which is the site of memory and learning. It’s also where Alzheimer’s plaques form.
Dr. David Holtzman, who is the chair of neurology at the Washington University School of Medicine in St. Louis, was also piqued. “It is obviously outside the box,” he said. “It really is an innovative and novel study.”
The researchers note that it is still unclear whether Aβ is combating a real or incorrectly perceived infection in AD. Regardless, these observations identify inflammatory pathways as potential new targets for drugs treating AD.
Sources:
PLOS ONE,
New York Times,
Science Translational Medicine