In
new research published in the journal Angewandte Chemie, scientists applied a high throughput methodology to study the ribozyme in an innovative way. While work in this area has typically used mutations to study the structure and function of the molecule, this new approach has applied multiple mutations at the same time.
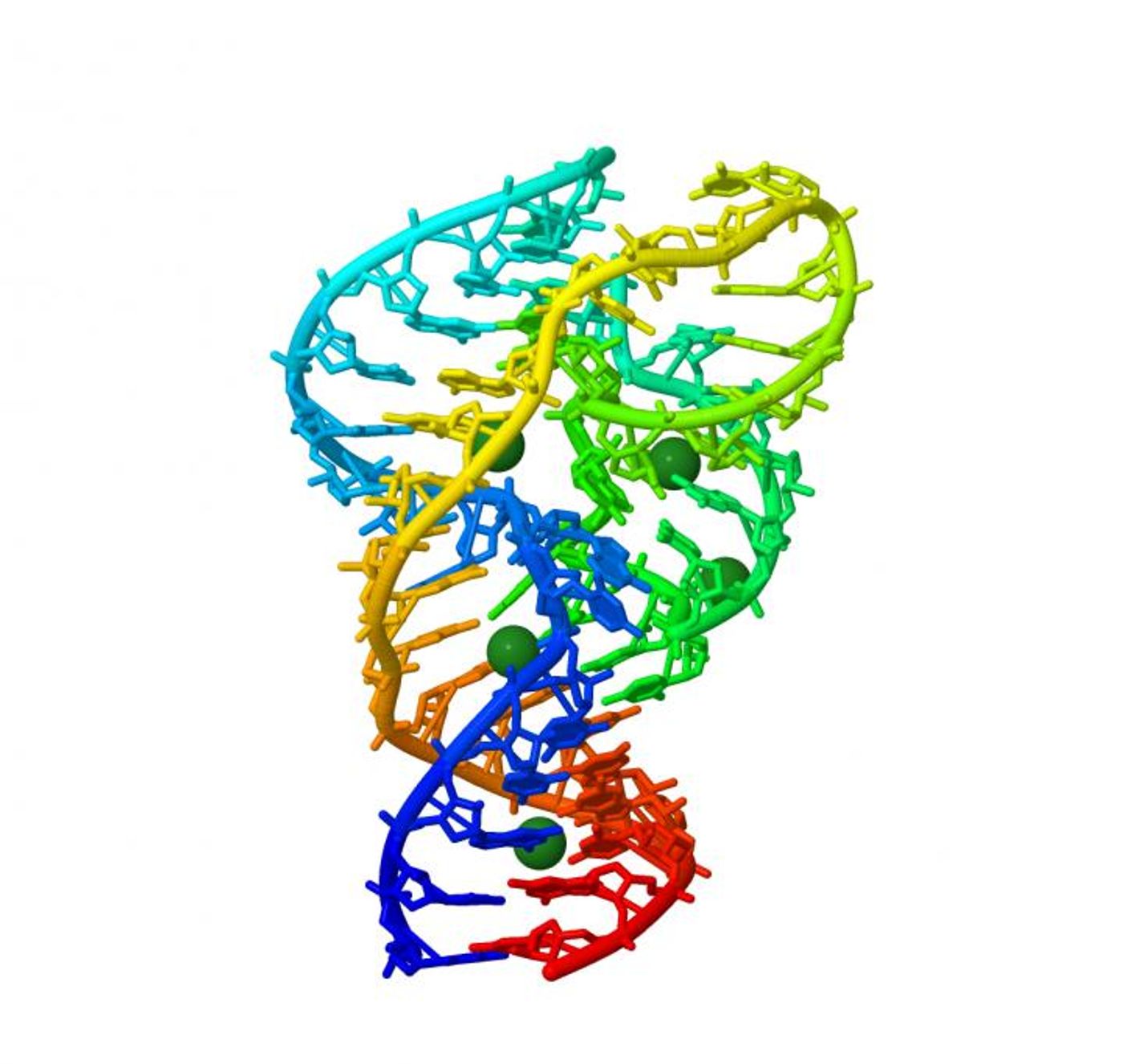
Ribozymes were discovered over 30 years ago, and more recently these ribonucleic acid structures have been found in myriad organisms. These molecules have not yet been fully characterized and all its roles are not yet known; they catalyze chemical reactions and are capable of joining amino acids, splicing RNA and replicating viral material. Some researchers have suggested that they performed many of the functions of enzymatic proteins before proteins evolved.
You can find out more about them by watching the video below, featuring Dr. Thomas Cech. He won the Nobel Prize for Chemistry in 1989 with Sidney Altman "for their discovery of catalytic properties of RNA."
Researchers that use mutations to reveal more about the structure and function of ribozymes can only use a few different modifications at any one time with current techniques. With all the different potential combinations of mutations, the selection process is often arbitrary, and such a method can thus miss important areas of the ribozyme sequence that could be critical clues to functionality.
Scientists at the Nucleic Acid Chemistry and Engineering Unit of the Okinawa Institute of Science and Technology Graduate University (OIST), Shungo Kobori and Yohei Yokobayashi, have created a brand new way of studying ribozyme mutants.
"Instead of selecting specific mutations, we decided to make and test as many mutants as possible of a specific ribozyme," explained Yokobayashi. A ribozyme with only one different base from the original ribozyme sequence is a single mutant. A double mutant has two bases that are different from the original. For even the smallest ribozyme studied by the researchers, only 48 bases, there are 10,296 total single and double mutants.
"With the help of a powerful DNA sequencer at OIST, we generated and then measured the chemical activity of all the single and double mutants of a variant of the 'twister ribozyme', which is found in the genome of rice," said Yokobayashi. "Thanks to this comprehensive approach, we now have a better understanding of which bases are more important for this ribozyme activity."
One particularly interesting finding is that the ribozyme is very tolerant to mutations. "This result is surprising," Yokobayashi said, "because the ribozyme we studied has a quite compact and complex structure." While much of the sequence likely helps to maintain the structure of the ribozyme, a significant portion of the mutants still had ribozyme activity.
The retention of enzymatic ability in the face of mutation might have been an evolutionary advantage, and may explain why ribozymes are so widespread among different forms of life.
Because ribozymes can control gene expression in both live cells and viruses, a better understanding of how they work could lead to improvements in their therapeutic applications.
Sources:
Biologist,
NobelPrize.org,
Angewandte Chemie,
AAAS/Eurekalert! via
OIST