Heat shock proteins (Hsps) are a kind of protein that are important in normal metabolic processes, and also the cellular response to stress such as extreme temperatures or wounds. They are named according to molecular weight, such that Hsp70 is a 70 kilodalton protein. They function to maintain protein health and function and critically, prevent a dangerous buildup of damaged proteins like tau, synuclein and beta amyloid, which have been implicated in disease.
Researchers Rui Sousa, and Eileen M. Lafer, both Professors of Biochemistry at the School of Medicine of The University of Texas Health Science Center, San Antonio
published work in the journal Nature Structural & Molecular Biology that demonstrates how heat shock proteins break up protein complexes. Apparently, when Hsp70s attach to protein complexes, rather than simply binding to them, the Hsp70s collide with the complexes with a force great enough to dissolve them.
"No one knew how the heat shock proteins pull apart bad protein complexes," explained Sousa. "At the molecular level, everything is moving, colliding and bumping, and smashing into other components of the cells. We found that the system moves Hsp70s to where they are needed. Once this occurs, collision pressures pull things apart."
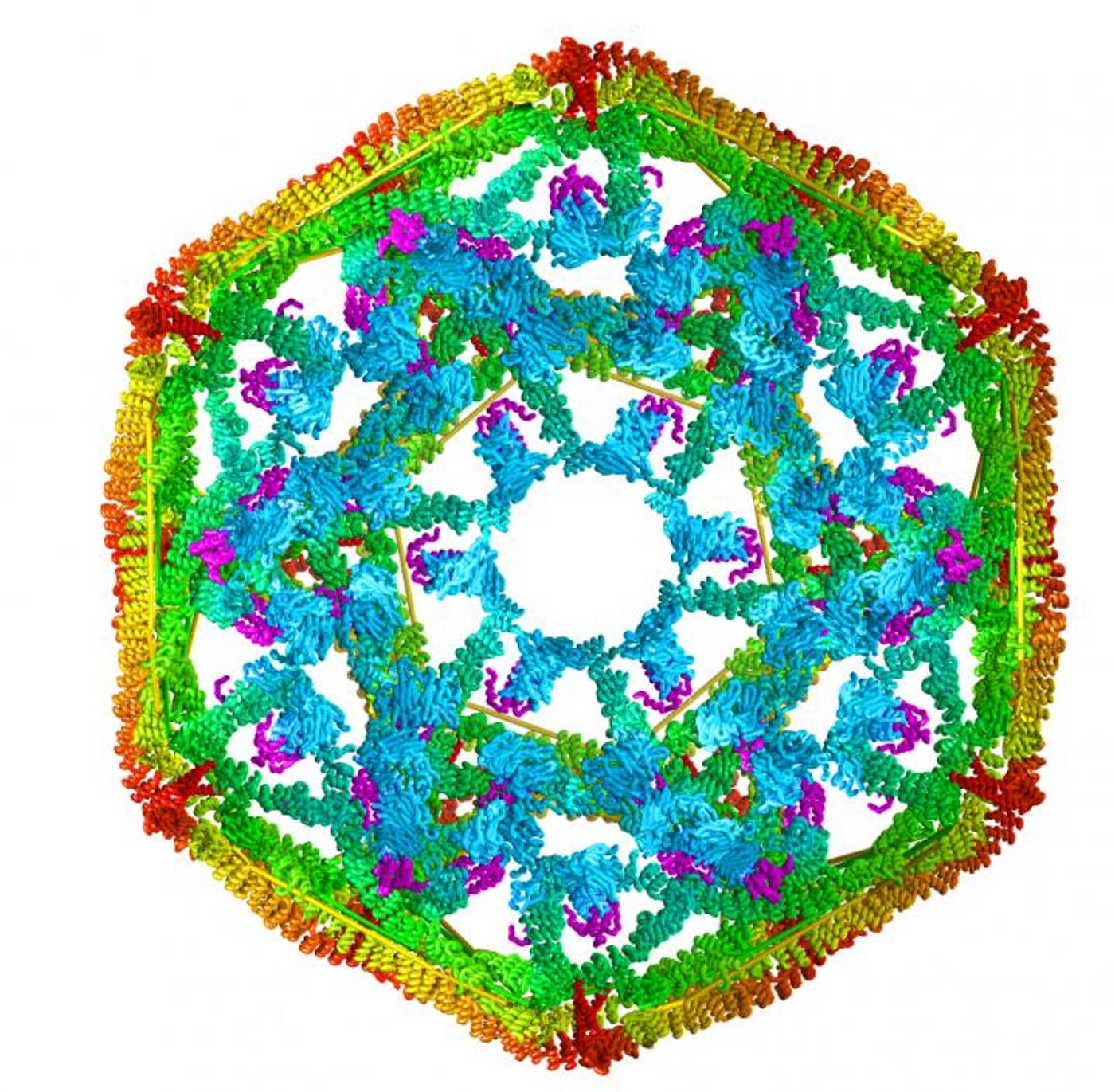
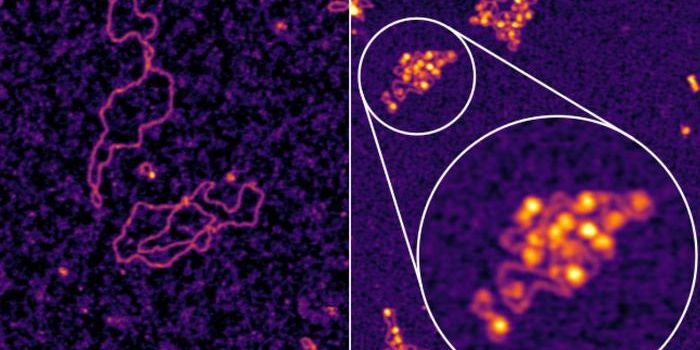
To tackle this work, the scientists had get around the fact that previous studies have been confounded by the use of too many different proteins for a precise characterization of the Hsp70 function. The researchers instead honed in on clathrin.
Clathrin is uniform in shape and size, and aids in the creation of intracellular cages that contain and move other proteins. Lafer made a technical breakthrough and succeeded in growing clathrin in bacteria (instead of relying on clathrin obtained from animals - another confounding factor). Now, the team was able to produce clathrin as needed for their work, and were also able to make genetic alterations to tailor it to their study and find the answers they sought.
"This work was a tour de force, requiring the convergence of exceptional biochemical and molecular genetic skills with a deep understanding of the principles of physical chemistry," commented Bruce Nicholson, Ph.D., the Chair of the Department of Biochemistry at the Health Science Center. "Such insights into the most basic aspects of protein chemistry and cell biology are often, as in this case, driven by a curiosity to find out how the molecular machines that drive our bodies work. But from these basic pursuits of scientific curiosity will often stem great benefits to human health."
"This is an impressive study that not only improves our understanding of cellular biology, but could lead to therapeutic discoveries for neurodegenerative diseases," said Francisco González-Scarano, M.D., Dean of the School of Medicine and Executive Vice President for Medical Affairs of the Health Science Center. "It is a tribute to scientists who ask hard questions and develop tools to answer them. My congratulations to the team."
"We attacked this problem because it was a really important question in cellular biology," Dr. Lafer said. "We didn't do it because we wanted to cure neurodegenerative disease or cancer. We know, however, that when we attack really important questions in science and biology, it ultimately leads to translational applications down the line."
"Sometimes as a scientist you just increase understanding of the way the world works," Dr. Sousa said. "This is something scientists have wanted to know."
Scientists have used upregulated Hsp70 function to cure Huntington’s disease in a fly model. Cancerous tumors also need Hsp70 to live. While the researchers remain modest about the applications, this is important work.
Would you like to know more about Hsps? The video above from Shomu’s Biology discusses them.
Sources:
Nature Structural & Molecular Biology,
AAAS/Eurekalert! via
UT Health Science Center