Researchers at Stanford University School of Medicine have found a critical link between all types of Parkinson’s disease. When a nerve cell fails to break down mitochondria that are no longer functioning properly, toxins are released into the cell. That failure is a common molecular characteristic seen in Parkinson’s.
“We’ve found a molecular biomarker that characterizes not just familial cases of Parkinson’s, in which a predisposition for the disease is clearly inherited, but also the condition’s far more prevalent sporadic forms, for which the genetic contribution is either nonexistent or not yet discovered,” explained the senior author of the work, Xinnan Wang, MD, PhD, an Assistant Professor of Neurosurgery.
Parkinson’s disease is the second most prevalent neurodegenerative disorder after Alzheimer’s disease, impacting roughly one in 65 Americans older than 65. Some cases run in families while most are sporadic. In the disorder, neurons that secrete dopamine fail and die, causing tremors, stiffness, difficulties with voluntary movement and sometimes cognitive dysfunction. There is a high degree of uncertainty surrounding the illness, with treatment impeded by lack of solid evidence.
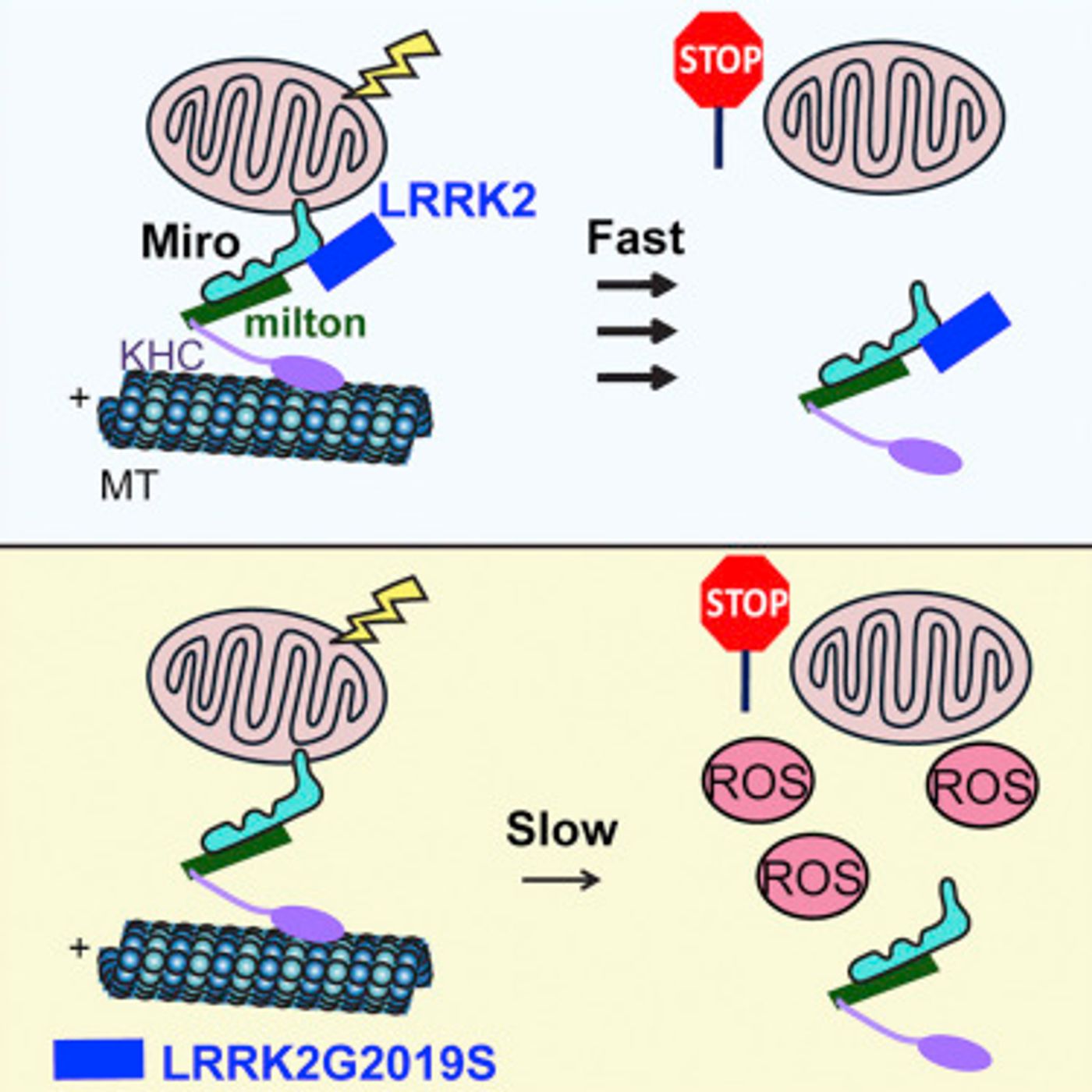
This investigation began with a gene, LRRK2, that has been linked to familial cases of Parkinson’s disease. The scientists discovered that a protein called Miro can only play its role in the removal of faulty mitochondria until it has formed a complex with LRRK2. Mutations in the LRRK2 gene impede the complex formation, leaving the malfunctioning mitochondria hanging around too long. When that happens, free radicals get released into the cell. The graphical abstract from
their research paper, published in Cell Stem Cell, is shown below.
Wang’s team utilized cell lines, specifically skin fibroblasts, that had been isolated from healthy people and Parkinson’s patients, several of which carried mutations in LRRK2 and some with mutations in other genes. When cellular events were monitored after mitochondrial damage induced by the researchers, it was observed that the detachment and breakdown of mitochondria was significantly delayed in all of the cell lines from Parkinson’s disease patients.
The researchers pursued their findings using microscopy to show that in the cell lines of healthy subjects, the damaged mitochondria of fibroblasts are quickly destroyed. In the Parkinson’s patient-derived lines, that process was appreciably slowed down.
The researchers also caused the production of excessive amounts of free radicals in the fibroblasts. Parkinson’s lines died as a result. “The healthy cells could handle higher free-radical concentrations,” Wang commented. “But the Parkinson’s cells were more prone to dying under those conditions, which are apt to occur in the energy-intensive, midbrain, dopamine-producing nerve cells that degenerate in Parkinson’s disease.”
In an impressive validation of their work, the investigators were able to prevent both cell death from free radicals as well as correct the delay in the removal of malfunctioning mitochondria in the Parkinson’s cell lines. They did this by lowering the levels of Miro in the cells.
“Existing drugs for Parkinson’s largely work by supplying precursors that faltering dopaminergic nerve cells can easily convert to dopamine, but that doesn’t prevent those cells from dying, and once they’ve died you can’t bring them back. Measuring Miro levels in skin fibroblasts from people at risk of Parkinson’s might someday prove beneficial in getting an accurate, early diagnosis. And medicines that lower Miro levels could prove beneficial in treating the disease,” Wang concluded.
If you would like to know more about Parkinson’s disease, check out the video above.
Sources:
Stanford News,
Cell Stem Cell