Anti-CRISPR Proteins can Reduce Off-target Effects
CRISPR-Cas9 is a gene editing tool based on the natural immune defenses of bacteria. While this new technique has been hailed as revolutionary, there has been some debate about its true potential for use as a therapeutic in humans because of unwanted side-effects. New research has found one way to reduce those undesirable outcomes, however. Scientists at UC Berkeley and UC San Francisco have demonstrated that there are proteins that can act to halt CRISPR-Cas9 activity once it's done its work; their work was reported in Science Advances.
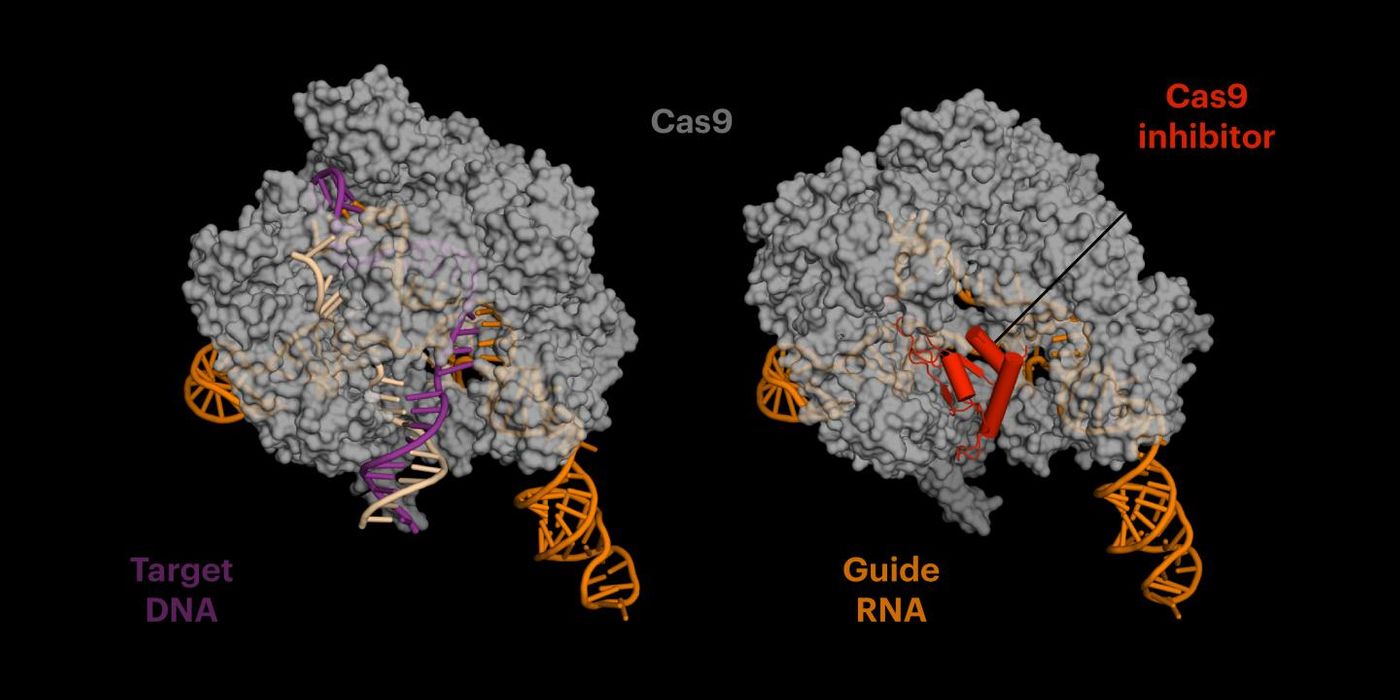
The CRISPR-Cas9 acts like a pair of molecular scissors, directed to a specific target in the genome where they make a cut. The inhibitor mimics DNA and binds to a site on the Cas9 protein, rendering it unable to make more cuts to the genome in areas outside of its target region. A protein discovered by co-author Joseph Bondy-Denomy, AcrIIA4 is being called an anti-CRISPR. When tested with a gene that causes sickle cell disease, it disabled the CRISPR-Cas9 and reduced off-target effects fourfold.
"Unexpected mutations can arise as a result of off-target gene editing, but our paper - like many others - shows that off-target effects can be modulated and it is not as serious as people might think," noted one of the first authors of the work, UC Berkeley postdoctoral fellow Jiyung Jenny Shin, from the lab of Jacob Corn at the Innovative Genomics. Shin used human cells in culture to determine that several hours after delivering the CRISPR-Cas9 system, the anti-CRISPR protein was the best way to decrease the number of off-target effects.
"Even after six hours of effective CRISPR, inserting anti-CRISPR decreases off-target effects by more than two-fold compared to on-target effects," Shin explained. "Therapeutically, you could treat a patient with CRISPR first, and then treat with anti-CRISPR at a later time and decrease off-target effects."
The Investigators suggest that anti-CRISPR proteins could become a routine part of CRISPR gene therapeutics; the kill switch might be delivered with the CRISPR-Cas9, halting gene editing after a certain time to reduce off-target cuts.
"This Cas9 inhibitor could be encoded on the same piece of DNA as Cas9, for example, precisely timed to turn Cas9 off after the gene editing is done, instead of letting Cas9 linger in the cell and risk off-target effects," suggested Bondy-Denomy.
Jennifer Doudna is one of the creators of the CRISPR-Cas9 technique. Members of her team had to figure out how anti-CRISPR and the CRISPR-Cas9 complex bind together. With cryo-electron microscopy, the scientists learned that because anti-CRISPR mimics DNA, CRISPR-Cas9 gets tricked into binding with it – a relationship that is permanent.
Bondy-Denomy reported finding four anti-CRISPR proteins last year. Viruses use them as a response to the bacterial CRISPR immunity; in this case, the proteins were inactivating a Cas9 protein of the Listeria monocytogenes bacterium. Two of the anti-CRISPRs also interrupted the Cas9 protein used most often in the lab, SpyCas9, from the bacterium Streptococcus pyogenes.
It has been suggested that there is an ongoing cycle between CRISPR-Cas9 and the repair mechanisms of cells: the Cas9 enzyme makes a cut at a target, and then the cellular repair machinery moves in to fix this new error, which is then snipped by the Cas9 again. A cut has to cause a mutation in the genome that prevents additional binding and more cuts. The CRISPR-Cas9 is then free to move to another part of the genome, looking for another binding site. An anti-CRISPR would help stop that vicious cycle in the hopes of halting damage to the genome in areas outside the target region.
"The ability to turn Cas9 gene editing off is just as important as the ability to turn it on," noted Corn, Scientific Director for Biomedicine of the IGI and a UC Berkeley Assistant Adjunct Professor of Molecular and Cell Biology. "Imagine if you had an electric razor with no off-switch! For eventual therapeutic applications, it is critical to be able to precisely control when and where gene editing is active. The anti-CRISPR proteins offer opportunities to completely turn off Cas9 as well as fine-tune its activity."
"Jenny's data suggests that there is an ideal time window for letting Cas9 do its job and then turning it off after that amount of time has passed," Bondy-Denomy said. "We can actually use the anti-CRISPR proteins as tools to figure out what that time window is, that is, for any one cell type with any one guide RNA sequence, how long we want Cas9 to be active in the cell."
In the video, biologist Neville Sanjana of New York University attempts to explain CRISPR technology to people of various ages.
Sources: AAAS/Eurekalert! via UC Berkeley News, Science Advances