With an estimated value at $9 billion,
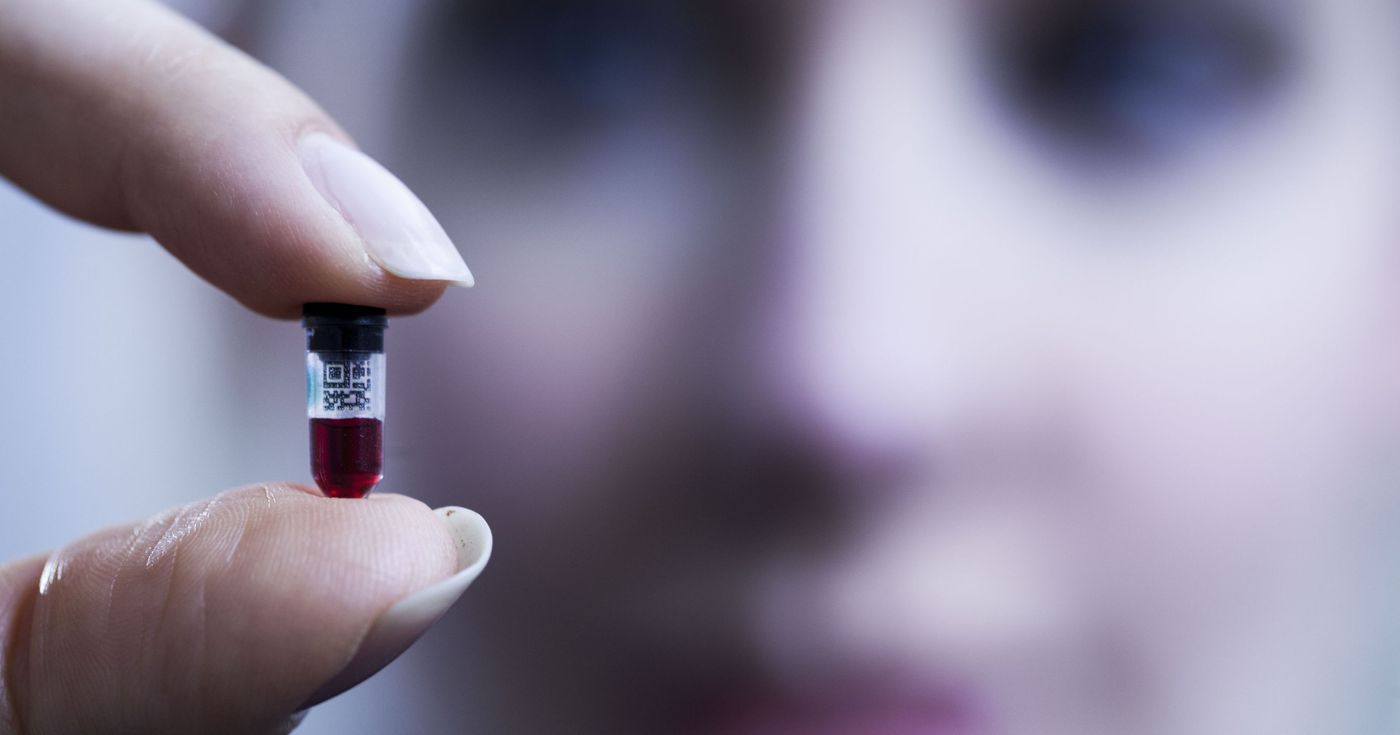
Theranos is, by all rights, not an average multi-billion dollar biotech startup. Going completely against the notion of “bigger is always better,” the company was founded on the idea of blood testing using just a few droplets via a finger prick. They claimed that over 100 blood tests could be done on just a few blood droplets. Their “less is more” approach extended to costs per test, advertising over 150 tests offered at
less than $10.
In an era of increasingly exorbitant health-care costs, Theranos’ ideas were deemed revolutionary and drew in massive funding support by the venture capitalists. But the eager hype surrounding Theranos has recently shifted into rowdy discomfort.
In a damning
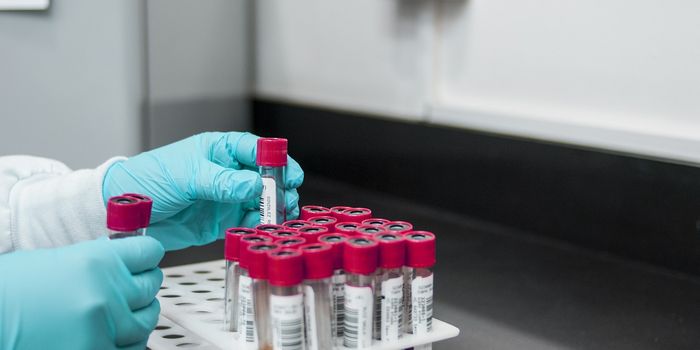
exposition published by the Wall Street Journal, Theranos was alleged to have stopped droplet blood collection via finger pricks for all but one out of the 240 tests it offers. The implication is that nearly all of Theranos’ blood collection is performed via traditional venipuncture, a method that collects blood in the volume of milliliters. If true, this stark contradiction could compromise the entire integrity of the droplet idea on which the company was founded.
As an even bigger cause for concern, results from blood test performed by Theranos are being seriously questioned for accuracy and legitimacy. In addition to independent reports of
discrepant test results, a recent
investigative report by the Food and Drug Administration (FDA) cited lack of quality audits for the medical device and laboratory tests offered by Theranos.
Another big deficiency cited by the FDA is Theranos’ unapproved use of the Capillary Tube Nanotainer (CTN), their proprietary blood collection device. While Theranos claimed the CTN to be a Class I device, the FDA classified it as a Class II Medical Device, requiring more rigorous regulatory and performance standards, which Theranos has yet to meet.
Arguably the challenges facing Theranos are complex, as this company is involved in two related steps of clinical diagnostics: it is the manufacturer of a clinical device, and it is a clinical laboratory provider of diagnostic services. This means that Theranos has a dual burden of complying with two regulatory bodies: the FDA and the Clinical Laboratory Improvement Amendments (CLIA). As the developer, manufacturer, and user of the CTN, Theranos must comply with FDA standards for the production and post-market surveillance of the CTN as a Class II medical device. In addition, as a clinical laboratory performing diagnostic tests, Theranos has to satisfy the Clinical Laboratory Improvement Amendments (CLIA) requirements for each of the clinical test it offers.
While there are some overlaps between the FDA and CLIA, Theranos must demonstrate compliance with both sets of regulations. And so far, Theranos’ FDA report card is less than desirable. Its CLIA report is yet to be made public.
With the controversies surrounding Theranos, would
you want the company to prick your finger? And would
you trust the accuracy of their test results? Walgreens, the U.S. drugstore giant, is having some
serious doubts, too. Amidst Theranos’ bloody storm, Walgreens has put a stop on the opening of additional Theranos Wellness Centers inside its pharmacies, stating that “Plans to open more Theranos Wellness Centers are dependent upon both companies’ ability to reach a mutually beneficial arrangement.”
Not surprisingly, the embattled founder and CEO of Theranos, Elizabeth Holmes, is fighting hard to regain control. She has rebutted all allegations and stood firm behind her company and its promises. But Theranos’ critics are not appeased. Is Theranos’ fall from grace imminent? Only time can tell. But one thing is for certain, with many questions still unanswered and FDA citations still fresh, the scrutiny on Theranos will only intensify.