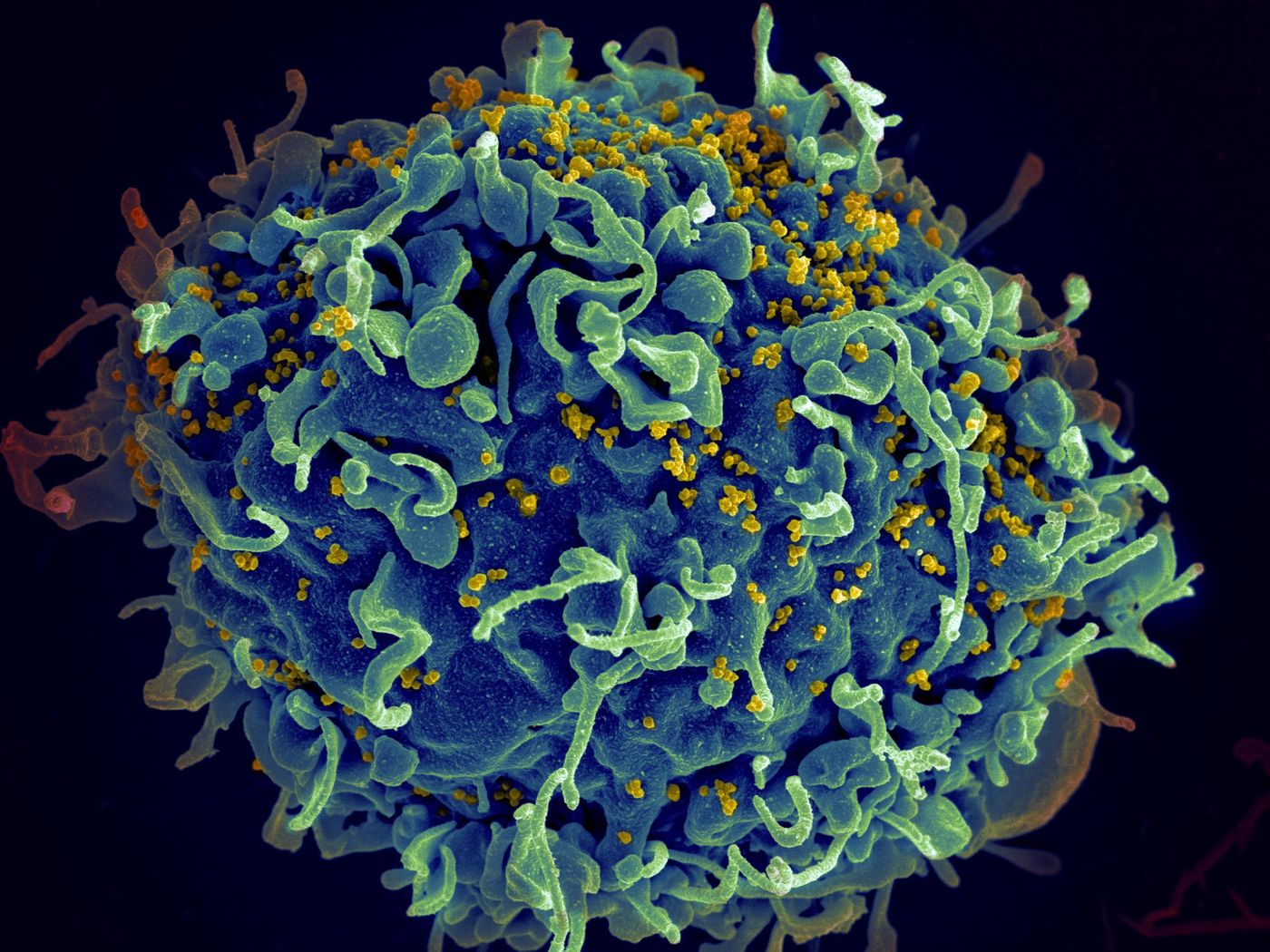
Human immunodeficiency virus, or HIV, is an infection that can lead AIDS when not treated. Unlike most viruses, HIV cannot be eradicated from the body completely. The virus attacks the immune system, especially a target a pollution of cells knowns CD4 (T-cells). These cells help our body fight off infections, and without their proper function, we are left vulnerable to opportunistic infections like cancers and commonly aids.
Unfortunately, no cure for HIV currently exists. However, approved medications can control the HIV infection through antiretroviral therapy (ART). But, overuse use of these medications produces a lack of efficacy when patients develop multidrug-resistant HIV infection.
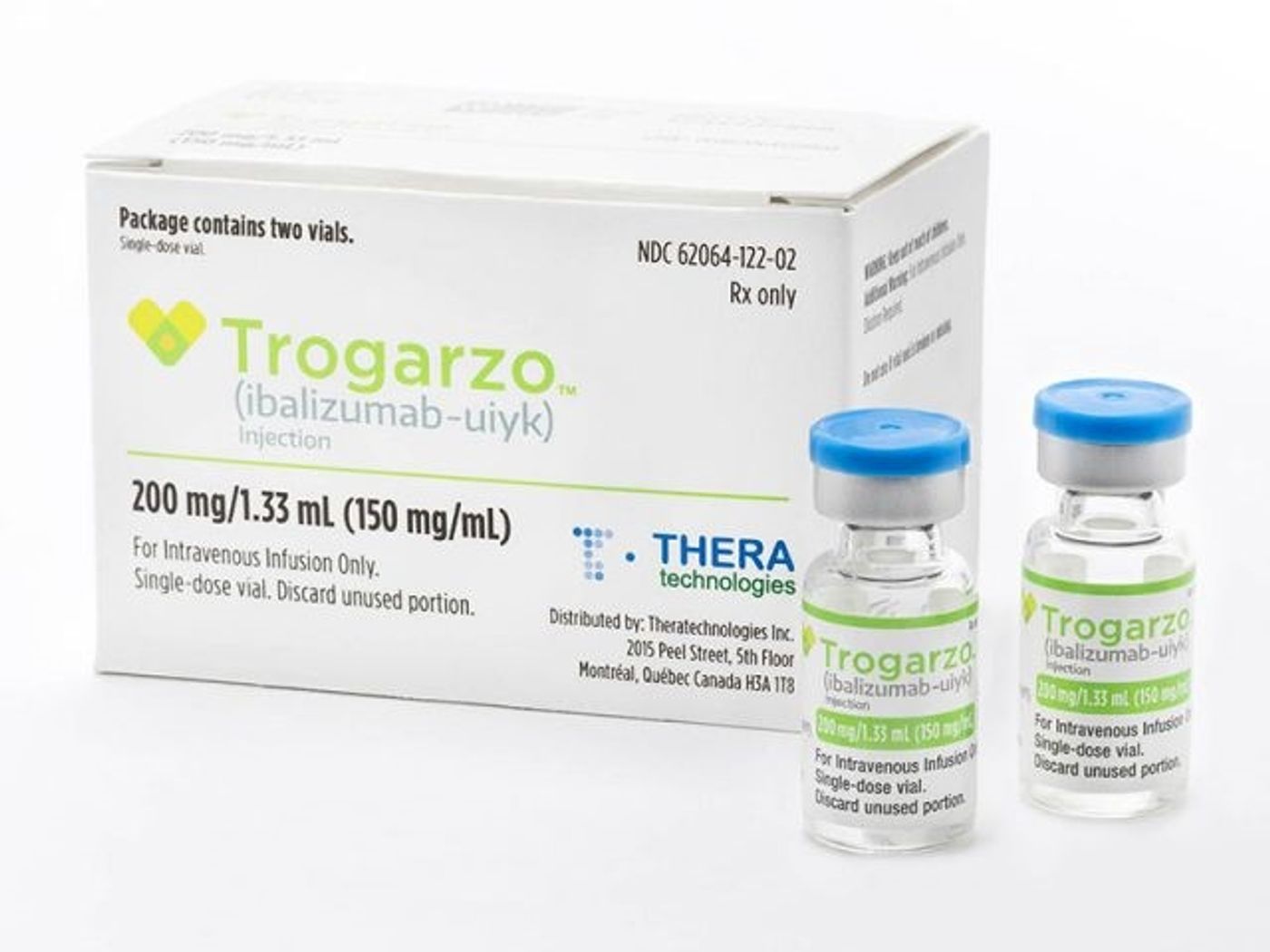
Now, the U.S. Food and Drug Administration approved a new treatment for patients living with multi-drug resistant HIV. The treatment is an antiretroviral medication known as Trogarzo, an ideal candidate for HIV infections that cannot be eradicated with other multi-drug resistant therapies (such as MDR HIV).
Trogarzo must be delivered intravenously once every two weeks by a licensed health care professional and in combination with other antiretroviral therapies. “While most patients living with HIV can be successfully treated using a combination of two or more antiretroviral drugs, a small percentage of patients who have taken many HIV drugs in the past have multidrug resistant HIV, limiting their treatment options and putting them at a high risk of HIV-related complications and progression to death,” says Jeff Murray, M.D., the deputy director of the Division of Antiviral Products at the FDA.
Image via Fronline Genomics
Trogarzo was examined to be safe and effective in series of clinical trials in individuals with strong multi-drug resistant HIV. These individuals continued to have high levels of the HIV RNA despite being on the therapeutic antiretroviral treatments. However, when these HIV drugs were taken in combination with Trogarzo, levels of the virus significantly decreased just one week after administration. “Trogarzo is the first drug in a new class of antiretroviral medications that can provide significant benefit to patients who have run out of HIV treatment options. New treatment options may be able to improve their outcomes.” The clinical trial participants continued to see improvement with administration of the bi-weekly regimen, Trogarzo plus antiretroviral drugs, and in 24 weeks results concluded HIV RNA suppression in participants.
The mechanism of action of Trogarzo works as an inhibitor that prevents viral entry and fusion of HIV. It is a humanized monoclonal antibody that specifically blocks the HIV virus from infecting host cells by binding to a domain of the CD4+ receptor.
Furthermore, the trial focused on a small patient population with limited treatment options. The evaluation for development included urgency of treating HIV, individualizing drugs in the therapeutic regimen, and data on the safety of Trogarzo. The side-effects include nausea, diarrhea, and dizziness with severe cases of rash and immune reconstitution syndrome.
Trogarzo is developed by TaiMed Biologics USA Corp.
Sources: U.S. Food and Drug Administration, Centers for Disease Control and Prevention