Digging Into the Details of DNA Replication
Cells have to carry around a massive amount of genetic material, and usually, that DNA is about 1,000 times longer than the cell where it lives. It can’t just be jammed in there though; it has to be carefully organized so that when various parts of it are needed by the cellular machinery, it is accessible. The DNA gets twisted and compacted, coiled again and again into a supercoiled structure. When cells divide, all that material has to be uncoiled so the cell can replicate it. Researchers have been teasing apart the various steps in that process for many years, building on that knowledge bit by bit.
DNA exists as two strands that are twisted into a helix, and when it is replicated those strands have to be pulled apart. The separation occurs in isolated spots, so as those pieces are uncoiled, it causes other areas to tangle more, building tension in the molecule. Special molecules ease that tension; called topoisomerases, they snip and twist as the strands separate, and are then rejoined. It had been thought that only topoisomerases were doing that job. New work from a collaboration between MIT and the Duke University School of Medicine has found, however, that topoisomerases are getting by with a little help from protein friends that move in when DNA is getting too twisty.
"We've known for a long time that topoisomerases are necessary for replication, but it's never been clear if they were sufficient on their own. This is the first paper to identify a protein in bacteria, or eukaryotes, that is required to localize topoisomerases ahead of replication forks and to help them do what they need to do there," said senior author Michael Laub, an MIT professor of biology and Howard Hughes Medical Institute Investigator.
To learn more about how topoisomerases were working, the team focused on the type II class of topoisomerases, which are found in a common bacterium, Caulobacter crescentus. Some antibiotics target type II topoisomerases used by bacteria, preventing bacterial DNA from replicating and thus cutting off the generation of new microbes, while not harming human cells. These drugs are used to treat tuberculosis, for example.
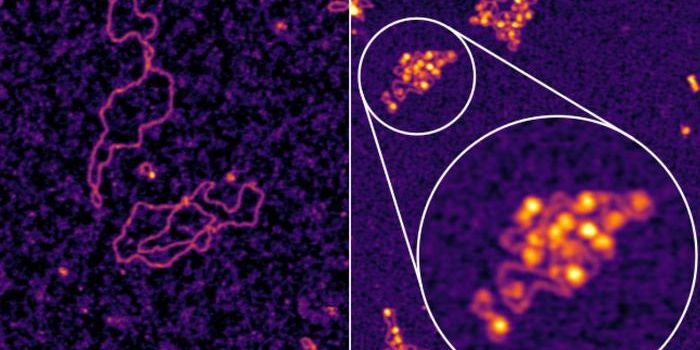
In higher order organisms, topoisomerase activation is controlled by other proteins. In this work, the researchers looked for proteins that were binding to DNA that was supercoiled; they would probably be involved in compacting that DNA, and maybe working with topoisomerase. That screen revealed a protein called GapR. When they removed that protein from the bacterium, they found the GapR was required for DNA replication. Without it, bacterial DNA twisted too much and replication was impeded; the bacterium died eventually.
The scientists found that GapR was recognizing overtwisted DNA, not specific sequences in the genome.
"The vast majority of DNA-binding proteins localize to specific locations of the genome by recognizing a specific set of bases," Laub explained. "But GapR basically pays no attention to the actual underlying sequence; just the shape of overtwisted DNA, which uniquely arises in front of replication forks and transcription machinery."
At Duke, a team led by Maria Schumacher elucidated the crystal structure of the protein while it's bound to DNA. GapR acts as a clamp on the DNA backbone, not the bases, when DNA is over twisted. When relaxed, the DNA doesn’t fit into that clamp, suggesting that GapR only sits on DNA when the topoisomerase is required. More work remains to reveal all the details of this process.
"In the absence of any other proteins, GapR is able to help type II topoisomerases remove positive supercoils faster, but we still don't quite know how," said postdoctoral fellow Monica Guo. "One idea is that GapR interacts with topoisomerases, recognizing the overtwisted DNA and recruiting the topoisomerases. Another possibility is that GapR is essentially grabbing onto the DNA and limiting the movement of the positive supercoils, so topoisomerases can target and eliminate them more quickly."
While this work started bacteria, it may also apply to eukaryotes, suggested Guo. "This was the first demonstration that a topoisomerase activator is required for DNA replication," she said. "Although there's no GapR homolog in higher organisms, there could be similar proteins that recognize the shape of the DNA and aid or position topoisomerases."
Sources: Phys.org via MIT, Cell