Unlike bacteria, viruses spread through and infect the human body by inserting viral DNA into our own cells, using them as a homebase to replicate their viral machinery. Scientists previously thought that viruses packaged their genetic material by pushing the DNA into a host cell with “lever-like” motions, but a collaborative new study from the University of Pennsylvania, the Georgia Institute of Technology, and Columbia University provides evidence for a different mechanism.
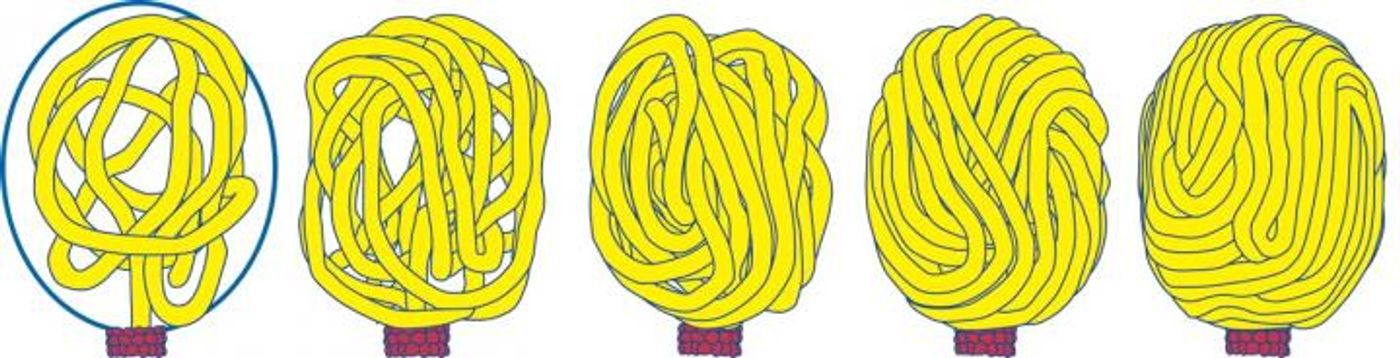
A new theory called the “Scrunchworm Model,” proposed initially by current team leader from Pennsylvannia, Stephen C. Harvey, PhD, last year, suggests that rather than being passive during their shuffle into host cells, viral DNA plays a more active role during packaging. Harvey’s idea revolves around biological motors that push genetic material into viral capsids with force generated by a repetitive cycle of DNA contracting and elongating, or “scrunching.” Studies have shown that DNA decreases in length by at least 25 percent after dehydration, and once inside the capsid the genetic material can be rehydrated and assume its full size.
To test the new model of viral DNA packaging, the researchers from Pennsylvania tested strains of phi29, a double-stranded DNA virus that infects bacteria, which is of similar structure to the herpesviruses, a family of pathogens that cause infectious mononucleosis, oral and genital herpes, chickenpox, and more.
Phi29 virus, on the other hand, is a
Bacillus bacteriophage with a protein-primed mechanism for DNA replication considered to be a model system. Phi29 is a member of the Podoviridae family, the smallest
Bacillus phages ever isolated, and overall some of the smallest-known double-stranded DNA phages.
They observed the interaction of DNA with phi29 connector proteins, which make up 50 percent of the protein motor, and tested them in computer simulations. They saw DNA spontaneous scrunching motions of DNA, with no sign of the so-called “lever-like” motions. The findings from this study provide the first-ever support for the “Scrunchworm Model.”
The researchers are hopeful that these findings will provide new ways for developing antiviral drugs, since they have unveiled an important mechanism double-stranded DNA viruses rely on for gene packaging. If antiviral drugs can block viral DNA packaging, an infection could be inhibited from spreading.
They already have an idea for a potential antiviral target based on this study’s findings. Using “lazer tweezers,” they believe they could latch on to the DNA tail of a single viral particle and prevent it from being packaged, like pulling on the end of a rabbit before it can disappear into a rabbit hole.
This study was recently published in
The Journal of Physical Chemistry B.
Sources:
University of Pennsylvania School of Medicine,
Medical Microbiology (4th Ed.),
Trends in Pharmacological Sciences,
Microbiology and Molecular Biology Reviews