Videos
The Animation of Antimicrobial Resistance
FEB 07, 2014 12:00 AM PST
Share
Cancer-Fighting Cells Transform to Repair DNA
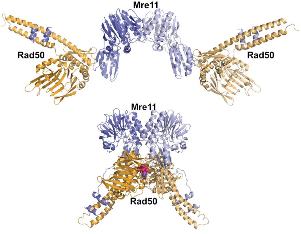 Did you know you have a form of shape shifting, cancer-fighting, transforming, DNA-repairing machines already hard at work throughout your body? No, it's not the mechanical devices from the Transformer movies or something out of a new science fiction novel; it's a protein complex known as Mre11-Rad50.
Did you know you have a form of shape shifting, cancer-fighting, transforming, DNA-repairing machines already hard at work throughout your body? No, it's not the mechanical devices from the Transformer movies or something out of a new science fiction novel; it's a protein complex known as Mre11-Rad50.Mre11-Rad50 is a versatile handyman of the cellular world, detecting and repairing the most seriously damaged sections of DNA. When both strands are cut in a DNA double helix, this protein complex attaches to the loose ends of the broken DNA, inhibits the continued division of the cell, and determines and enables the correct repair method to fix the DNA strands. This is a high stakes operation for the cell - a double break in a DNA strand is fatal to the cell, but an incorrect repair could enable the growth of cancerous cells.
While it's known that the ability to modify its shape plays a role in the DNA-repairing process, not much is known about how the two are related. Two recent studies supported by the National Institutes of Health (NIH) and the National Cancer Institute focused on the mechanism of Mre11-Rad50 and how it performs its DNA-repairing tasks.
The concept in both studies was to modify the structure to address specific functions of Mre11-Rad50. The team was then able to test the effect of the modification on Mre11-Rad50's ability to change conformations and the rate at which it changes, and in turn, the effect on its ability to repair DNA.
The conformational effects were studied using the SIBYLS (Structurally Integrated Biology for Life Sciences) beamline at the ALS (Advanced Light Source) at Lawrence Berkeley National Labs. This system uses high-intensity X-rays to get the combined effect of x-ray diffraction and X-ray scattering, resulting in images of the protein's crystal structures with atomic level resolution. More importantly, the scientists can see the conformational changes of the protein as they take place.
In the first study, recently published in Molecular Cell, the research team included molecular inhibitors that affected the ability of the Mre11 component of the protein complex to cut DNA and watched the effect on the DNA repair process with human cells. The assumption was that Mre11 initiated the repair process at the broken end of the DNA, but in this case the team discovered that Mre11 removed a small portion away from the broken end and started a DNA-repair pathway known as homologous recombination repair - working back toward the broken strand of DNA instead of starting there.
In the second study, recently published in EMBO Journal, the Rad50 component was modified. This research team found that mutations could be created that modified the binding of Rad50 with ATP, causing ATP hydrolysis to either slow down (in turn promoting a non-homologous end-joining repair method) or speed up (promoting a homologous repair method). The team was able to alter the repair path through modification of the base Rad50 structure.
A better understanding of the Mre11-Rad50 mechanisms can lead to more targeted cancer treatments in the future, as well as other applications in the life sciences. Perhaps a series of targeted, transforming cell therapies are in our collective future.
Sponsored by

You May Also Like
Loading Comments...








