We understand microglia and inflammation but to think they play a critical role in pain is new. When in pain neurons transmit the information, but the purpose of non-neuronal cells in pain is gaining more momentum. Now we understand that when something terrible happens in the body microglia get activated but understanding the role of microglia in pain can be complicated. A little porthole into this neuroimmune world begins with understanding how microglia are different in pain vs. no pain state.
P2X4, a receptor in microglia is overexpressed after peripheral nerve injury; this is the first evidence linking glial cells to pain back in 2003 by Tsuda et al., Same year, Jin et al., showed that spinal microglia play a role in pain hypersensitization post peripheral nerve injury by activating the p38 MAPKs. As of 2018, approximately 40 microglial associated proteins have been identified that are modulated due to pain and cause hypersensitization. Additionally, enabling the microglia in non-injured conditions also result in hypersensitization, and this led to coining a new term “microgliopathic pain.”
Microglial are not static cells
Microglia don’t stay put in one place; their processes are constant searing the microenvironment to relay the health. While it is still not understood how this works, peripheral nerve injury had been shown to reduce the length of the processes, increase in volume as soon as a day after the injury. It is later seen that microglial number increases post nerve injury. Davalos et al., work published in 2005 Nature Neuroscience showed the dynamic microglial response to traumatic brain injury via two-photon imaging.
“Microglia are the gossipers that amplify the pain,” says Linda Watkins, Ph.D., Distinguished Professor, Department of Psychology & Neuroscience and Center for Neuroscience, University of Colorado, Co-Chair, Advisory Board, Xalud Therapeutics, Inc.
Priming Microglia
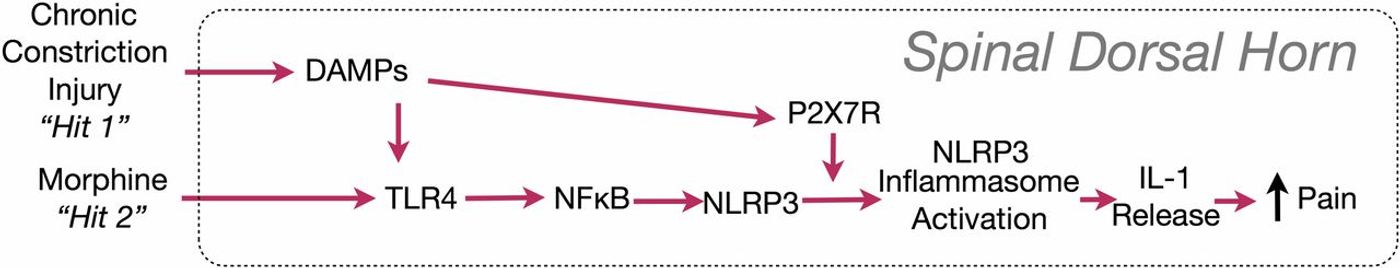
Glial cells are not just on and off alone. The third state exists primed state they respond faster stronger and longer than before. A study published in PNAS 2016, by Peter M.Grace describes the microglial “priming.” The findings support a “two-hit hypothesis” of amplification of microglial activation—nerve injury being the first “hit,” morphine the second. This new understanding shed new light on opioid-induced pain sensitivity. Opioid administration causes glial activation, which paradoxically opposes the pain-modulating effect of opioids. Reversal of the pro-inflammatory activation of glia would thus not only reverse neuropathic pain in general but should also enhance the clinical efficacy of morphine and other opiates.
Image Source: Grace et al. 2016 PNAS
Gene Therapy for Pain
Glial cells are activated by both isomers of drugs and antagonists. Whereas neurons only get activated by positive isomers. So therapeutic approaches, that block only glia but not neurons could prolong the analgesic effects.
Gene therapy treatments for neuropathic pain are a focus of Xalud Therapeutics. The company is developing XT-101, a single administration therapeutic designed to reverse glial activation. XT-101 is an intrathecal therapy that drives the production of the anti-inflammatory cytokine interleukin-10 (IL-10) at the spinal cord.
Dr. Watkins started to understand the glia in 1991 and 2018, the company she founded Xalud Therapeutics has a drug approved for clinical trials. An impressive culmination of glial pain journey! To know more watch Dr.Watkins in action.