Approximately 468,000 individuals are on dialysis for kidney failure. Dialysis is a life-saving procedure, but it is not without its complications. One issue with dialysis is how to get the blood in and out of the patient’s body. The most popular and the safest way to do this is with an arteriovenous (AV) fistula, which is a surgically-created connection between an artery and a vein. Over 50% of patients on dialysis use an AV fistula because it has the least amount of issues with infection and clotting compared to the alternatives. The two alternatives to AV fistula are an expanded polytetrafluoroethylene (ePTFE) graft and a catheter. Less than 30% of dialysis patients use a graft and about 18% use a catheter. While catheters have the most problems with infection, grafts also have more issues with clotting and infections than AV fistulas. Unfortunately, some patients are unable to have an AV fistula or the fistula failed so they have to use one of the less desirable alternatives. The more popular alternative, the ePTFE graft, has the additional issue that it is not very durable. Better options are needed for the hundreds of thousands of people who rely on dialysis.
Thankfully, regenerative medicine has advanced enough to created an alternative. A big, collaborative effort between scientists at Yale and Duke and doctors in Poland and the U.S. has created a bioengineered blood vessel that is safe, effective, and more durable than the synthetic grafts.
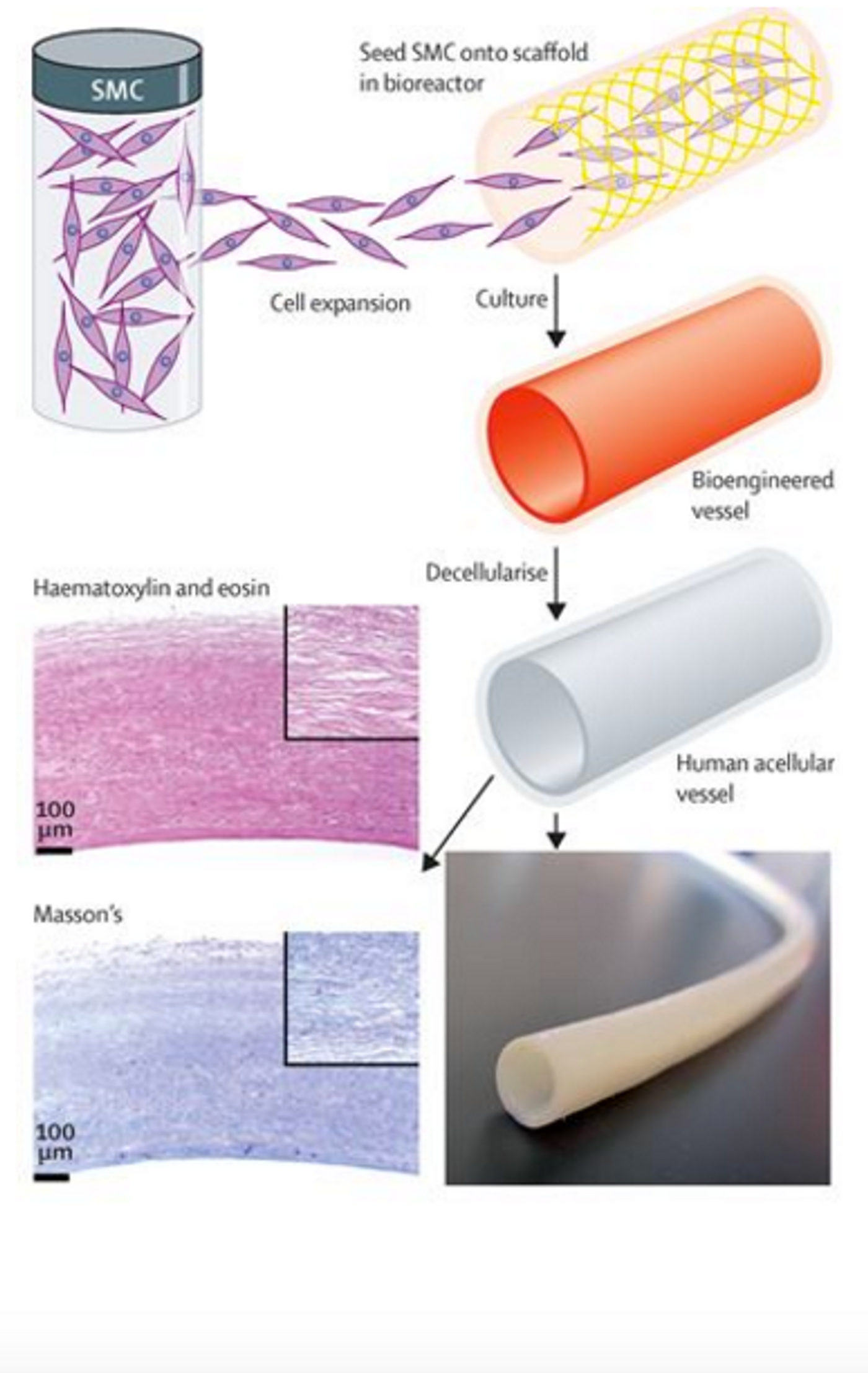
The science and the techniques behind this creation are remarkable. The first step is isolating vascular cells from a human donor and growing them in tissue culture. The cells are then placed on a degradable scaffold in the shape of a blood vessel where they will grow and stretch and eventually acquire the physical properties of a real, functional blood vessel. This part of the process takes about 8 weeks. After the 8 week growing process, the scaffold disintegrates, leaving behind a blood vessel. You might think that this is where the process stops, but the researchers took it further. The final step in the process is to wash away all of the cellular, living components to leave behind a protein structure that is mostly collagen. This protein structure maintains the blood vessel shape and is ready for implantation.
The fact that this engineered blood vessel is acellular is crucial because it minimizes the risk of rejection by the patient. In the 60 patients who received the bioengineered blood vessels, there was no incidence of rejection. Because the blood vessels are acellular, the patient's’ cells actually repopulated the collagen scaffold after it was implanted. Durability 1 year after implantation was 90% for the bioengineered blood vessels compared to 60% for the synthetic grafts and the engineered blood vessel were just as safe.
This work will not only help those on dialysis who cannot have an AV fistula, but it also represents a huge step in the broader field of regenerative medicine. Nonliving bioengineered tissue has been implanted to becoming living tissue. This is a very cool advancement that can be applied to multiple areas.
Sources:
EurekAlert,
The Lancet, and
NIDDK









