Human Stem Cell Descendants: Drugs of the Future
Immune cells, like all cells of the human body, come from less-specific ancestor cells, sometimes called progenitor cells or stem cells, creating a family tree of sorts that resembles family trees we’re familiar with, decked with mothers and fathers, aunts and uncles, and other distant relatives. A new study from Tokyo Medical and Dental University (TMDU) is focused on understanding the birth of immune cell lineages, holding that this knowledge is the key to new protection from pathogens.
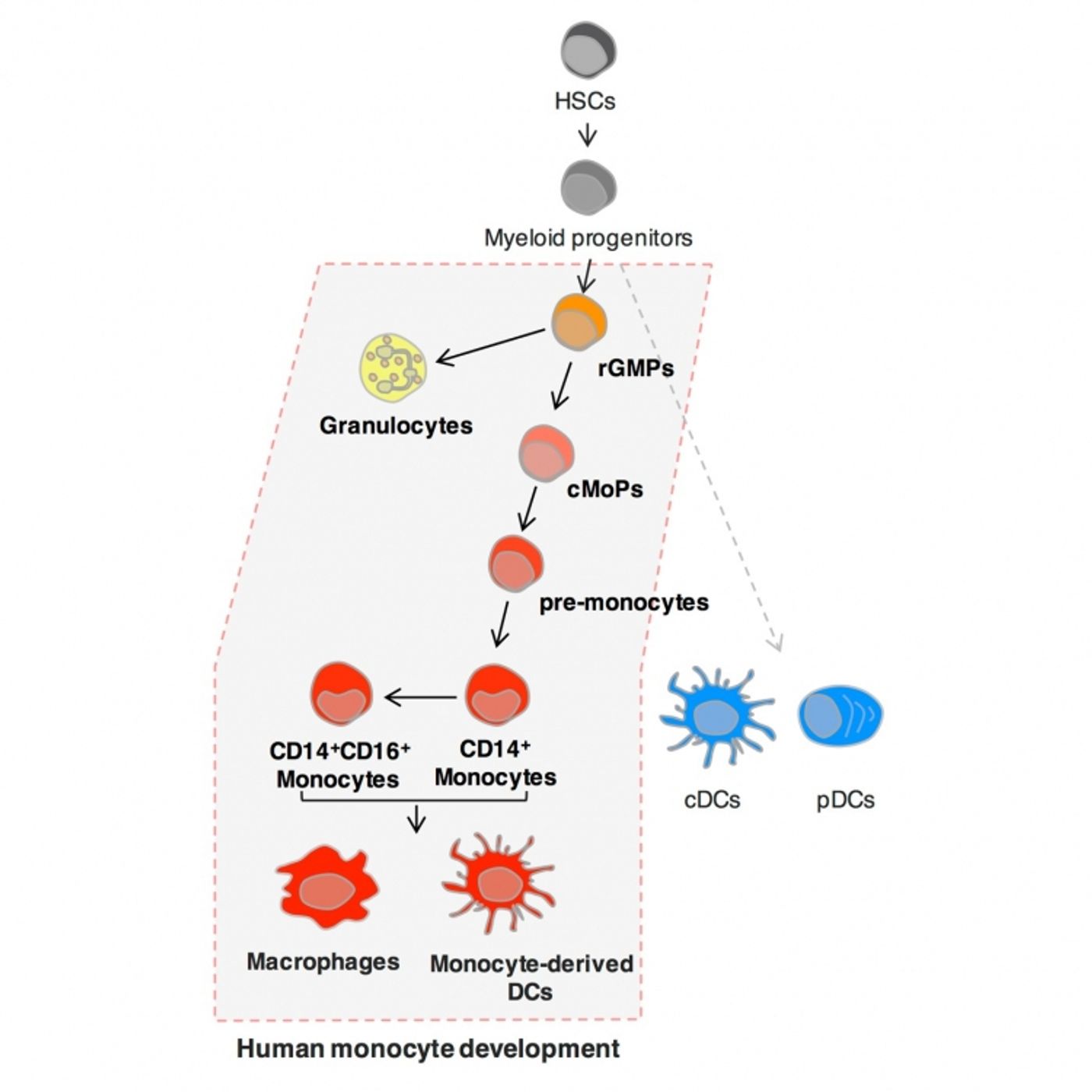
There are certain human stem cells, undifferentiated cells with the ability to become any cell type, that scientists believe are “destined” to become specific immune cells, called monocytes. Monocytes, produced by a common monocyte progenitor cell (cMoP), are precursors to specialized immune cells like macrophages and dendritic cells, which serve the immune system by engulfing pathogens and recognizing chemical signals expressed by pathogenic cells, respectively. However, monocytes also serve as a precursor to cells associated with disease, including tumor-associated macrophages, osteoclasts, and colitogenic macrophages. This makes monocytes a clear target in certain contexts.
Monocytes differentiate into macrophages and dendritic cells based on inflammatory signals that send messages about what the immune system needs most. Researchers first identified the precursor to monocytes, cMoPs, in mice, but now these ancestor cells have also but identified in humans.
How are certain monocytes created? How could scientists target cMoPs to increase the amount of monocytes produced to boost the immune response? How could scientists target cMoPs to decrease the amount of monocytes to prevent aiding diseases like cancer? Researchers from TMDU were and are devoted to answering these questions. In their study, the screened all of the unique proteins displayed by monocytes, macrophages, and dendritic cells, to distinguish how their expression influenced differentiation. Two proteins, CLEC12A and CD64, were found to mark the difference between high and low expression levels.
TMDU researchers created four subgroups according to expression levels of CLEC12A and CD64. For example, stem cells expressing high levels of CLEC12A and CD64 only differentiated into monocytes, suggested that these stem cells were the cMoPs previously identified in mice. High levels of CLEC12A and intermediate expression of CD64 instead led to granulocyte and monocyte differentiation. Those groups with no CD64 expression at all resulted in dendritic cells only.
"Focusing on the expression level and pattern of cell surface proteins, we observed a sequential differentiation process from granulocyte/monocyte progenitors via cMoPs to pre-monocytes then monocytes that was supported by gene expression studies," corresponding author Toshiaki Ohteki says. "This updates what we know about the human blood cell developmental pathway."
While the work of Ohteki and the TMDU research team seems far away from being applied to a treatment useful in the clinic, their research provides the sturdy scientific foundation needed for anti-cancer and anti-infection therapies of the future, those that defy all limitations as we know them.
The present study was published in the journal Immunity.
Source: Tokyo Medical and Dental University