A specific group of antibodies are nurtured by the immune system to fight HIV. This process naturally occurs in the presence of HIV and takes two years. In order to speed up the process and to start effectively mounting counterattacks against HIV infections, scientists look to specialized vaccine creation.
Antibodies from the PGT121 family were recently discovered to neutralize HIV in the body through a complicated series of interactions (
PLOS Pathogens). In a recent study by scientists from the Scripps Research Institute (TSRI), a team looked specifically at the action of PGT122 and PGT124, two members of the PGT121 family that act as broadly neutralizing antibodies, meaning they can stop “many strains of the rapidly mutating virus.”
PGT122 and PGT124, like the rest of the PGT121 family, have a unique capacity for targeting the HIV envelope glycoprotein. This structure is composed of two subunits and has been an HIV drug target for many years (
Current Opinion in Structural Biology).
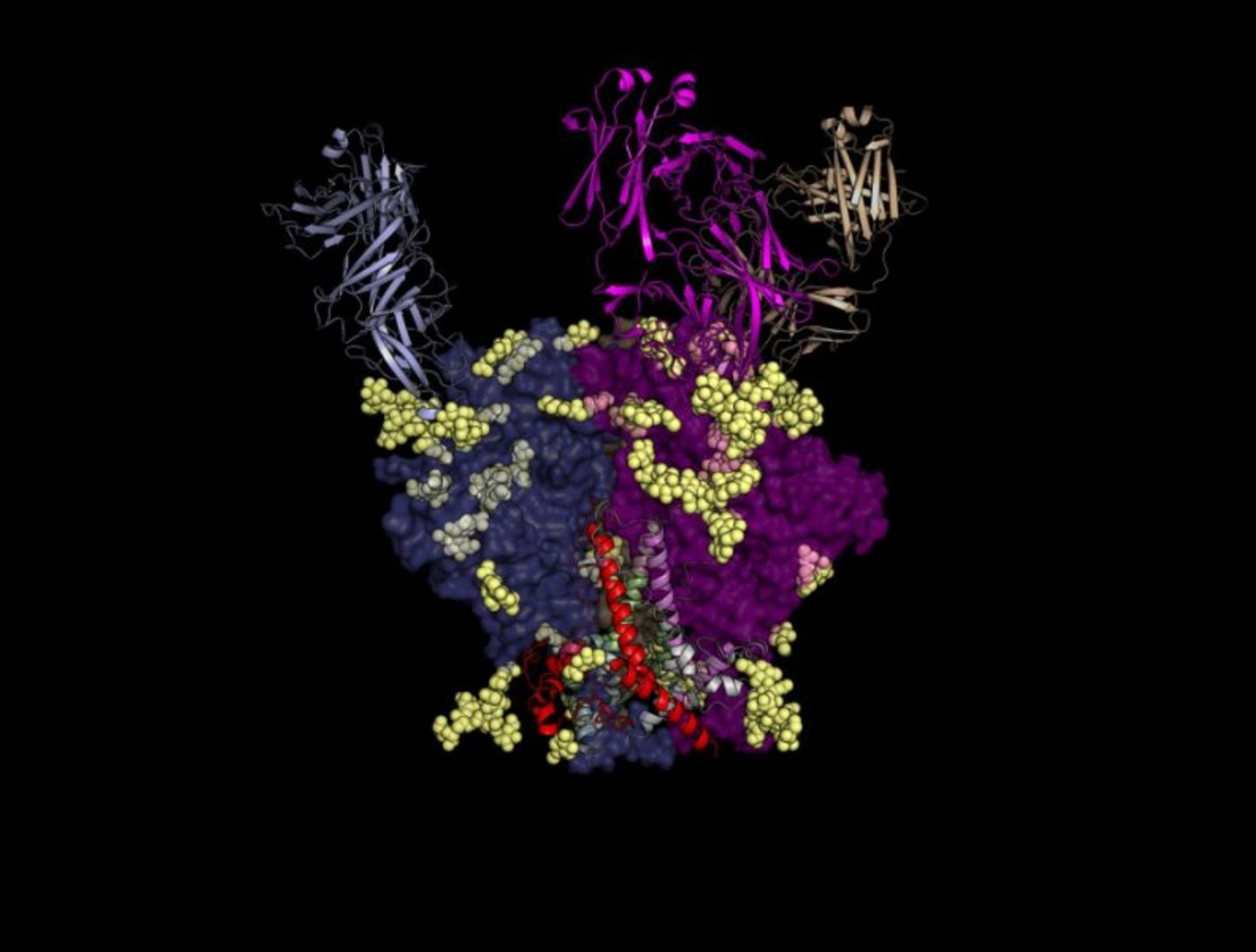
With the idea of mimicking their actions in an HIV vaccine, TSRI scientists “mapped out the structures” of the broadly neutralizing antibodies PGT122 and PGT124, examining the images at various stages in their differentiation.
"What makes the PGT121 family of antibodies so special," explained Fernando Garces, TSRI research associate and first author of the study, "is that it has found ways to counter-attack the virus while many other families of antibodies fail to do so."
Initial image results showed PGT122 and PGT124 binding the N332 glycan of HIV’s “glycan shield.” The continuously evolving proteins of the glycan shield enable the virus to successfully evade most neutralizing antibodies (
Nature Medicine). Thus, the glycan shield is the key target for PGT122 and PGT124.
While binding N332 glycan, PGT122 and PGT124 simultaneously bind a the GDIR motif of the envelope glycoprotein, a small piece of the glycoprotein that is conserved across 94.1% of HIV-1 strains (
Cell). TSRI scientists refer to N332 and the GDIR motif as “binding partners” because binding of both is required for effective viral neutralization.
From this point on, images show PGT121 family members pursuing different approaches in their HIV attack. PGT122 antibodies target the N137 glycan by mutating. PGT124 antibodies also mutate, but they attack HIV envelope glycoprotein underneath the glycan shield. TSRI scientists also saw that if the N137 glycan is scientifically removed from HIV, neutralizing antibodies from the PGT121 family “immediately behave as well-trained antibodies and don’t require a two-year boot camp.”
These observations provide ample insight into possible therapeutic strategies with vaccines by manipulating the activity of PGT122 and PGT124.
“If scientists can design a molecule that lacks this N137 glycan, they may be able to prompt a faster antibody response,” TSRI scientists said. “Once the antibody response has been kick-started, they could add this glycan back and try to mature the antibody against the more native form of the virus.”
The key behind this theory is understanding how the PGT121 antibodies change as they target HIV, explained Ian Wilson, PhD and Hansen Professor of Structural Biology at TSRI. The results of the study were published in
Immunity this week.
To learn more about the potential of broadly neutralizing antibodies in the realm of HIV vaccination, check out the following video.
Source:
The Scripps Research Institute