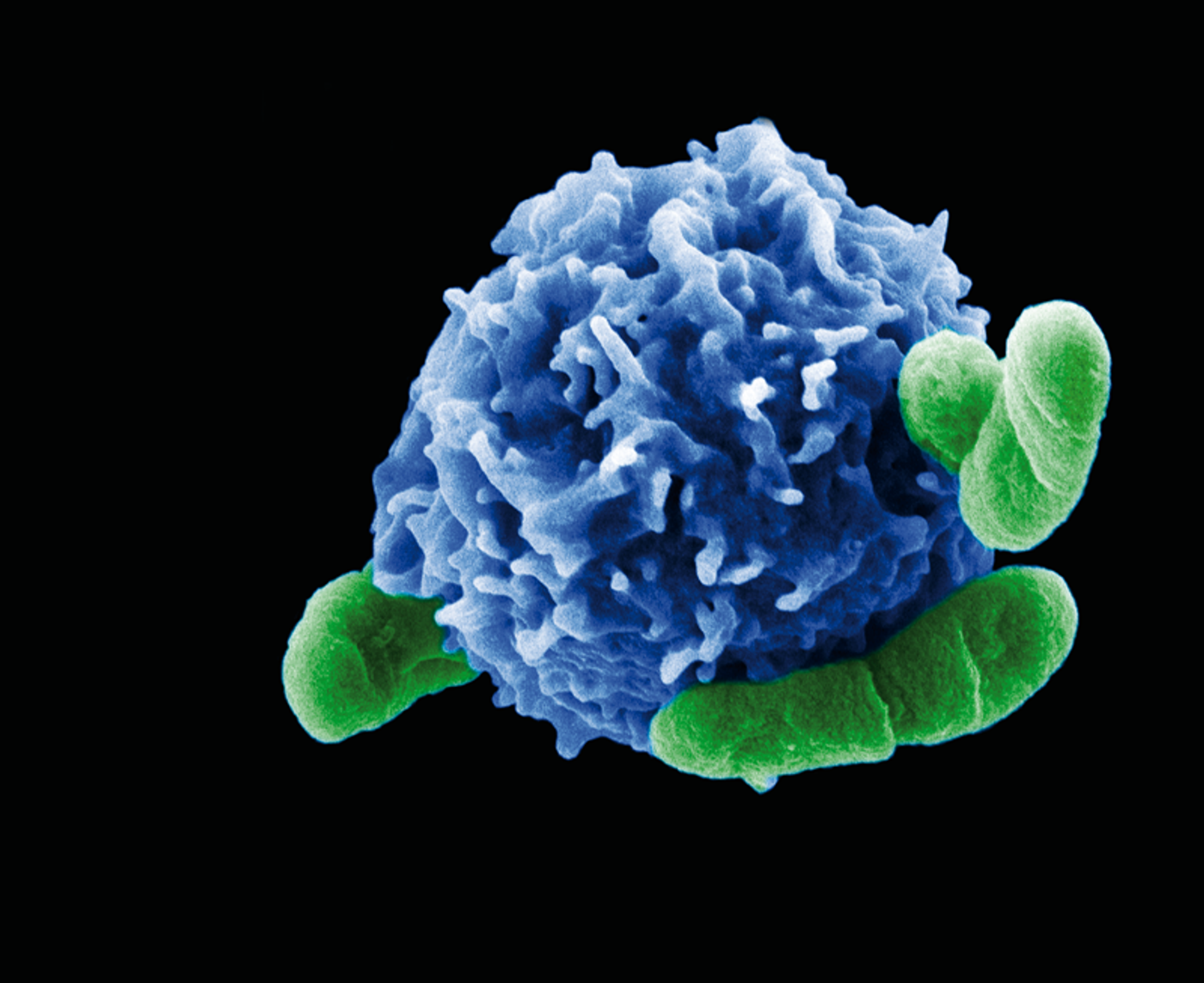
Acting alongside T helper cells, the all-stars of adaptive immunity, regulatory T cells work to suppress any “potentially deleterious” T helper cell activities that could lead to an autoimmune disorder (
Scandinavian Journal of Immunology). T helper cells activate effector immune cells to attack invading pathogens and abnormally growing cells, which is a good thing. However, these lymphocytes can also make mistakes and wrongly target the body’s own cells. This is where regulatory T cells come into play, protecting the body by maintaining self-tolerance and preventing allergic responses.
With regulatory T cells at the ready to keep the body’s immune system from attacking itself, other immune cells are free to concentrate on attacking pathogens and killing cancerous cells. However, what happens when the regulatory T cells themselves need protecting?
A new study published in
Nature Immunology by scientists from St. Jude Children’s Research Hospital investigated the protective mechanisms of regulatory T cells. Like a doctor who helps treat and prevent other people from getting sick, regulatory T cells also depend on certain cellular processes to continue normal function, similar to how doctors wear masks, gloves, and wash their hands when with a patient.
"Regulatory T cells are very specialized cells that require activation to perform their function in curtailing undesirable immune responses," said study leader Hongbo Chi, PhD.
After being activated, a process called “autophagy” relieves regulatory T cells of “molecular garbage” that piles up in the cell. Imaging studies done from colon cancer cells in Chi’s experiments proved the necessity of autophagy for regulatory T cell function. Next, the team looked at mice models of disease to see how organisms respond as a whole when the process of autophagy was disturbed. Key genes for autophagy, Atg5 and Atg7, were deleted, and they saw regulatory T cell malfunction as expected in these knock-out mice.
Activated regulatory T cells without functional autophagy experienced excessive cell death and a “loss of identity,” a phrase Chi used to describe their non-regulatory T cell action that occurred without autophagy. Thus, after losing their traditional abilities and gaining new ones, the regulatory T cell population in the knock-out mice were actually able to clear tumors better than control mice.
"From this perspective, targeting autophagy could act in synergy with strategies that block autophagy in tumor cells for added benefits in cancer therapy," Chi said.
He and his team plan to study other tumor cell types with autophagy inhibition in regulatory T cells, focusing on the “detailed biochemical mechanisms” behind the protective mechanism of autophagy for regulatory T cells. The potential for new immunotherapies for cancer to be derived from this research is great, but Chi and his team have to ensure that upsetting the protective function of regulatory T cells does not create dangerous backlash for cancer patients.
Source:
St. Jude Children’s Research Hospital