"Blood and Guts" Study Maps B Cells All Over the Body
What scientists are calling the B cell “clonal geography” has been the subject of a long project at the University of Pennsylvania School of Medicine. Researchers developed a new computational framework for analyzing the way B cells form networks in different tissues in the body. The framework is called “ImmuneDB.”
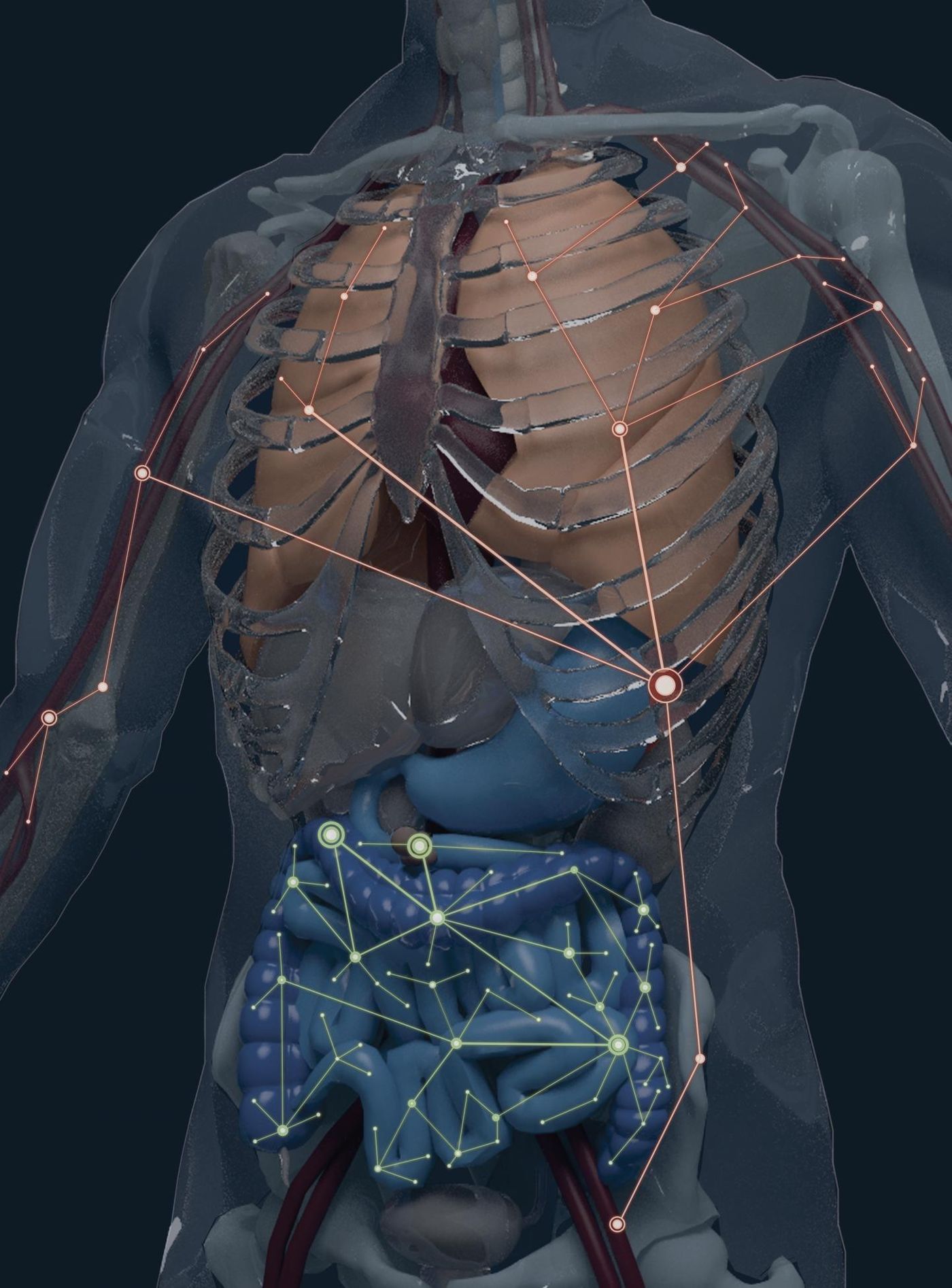
"We dubbed our study 'Blood & Guts,' when we started to see that B-cell clone populations partition into two broad networks,” explained senior author Nina Luning Prak, MD, PhD. “We essentially discovered and mapped the B-cell clonal geography of the human body.”
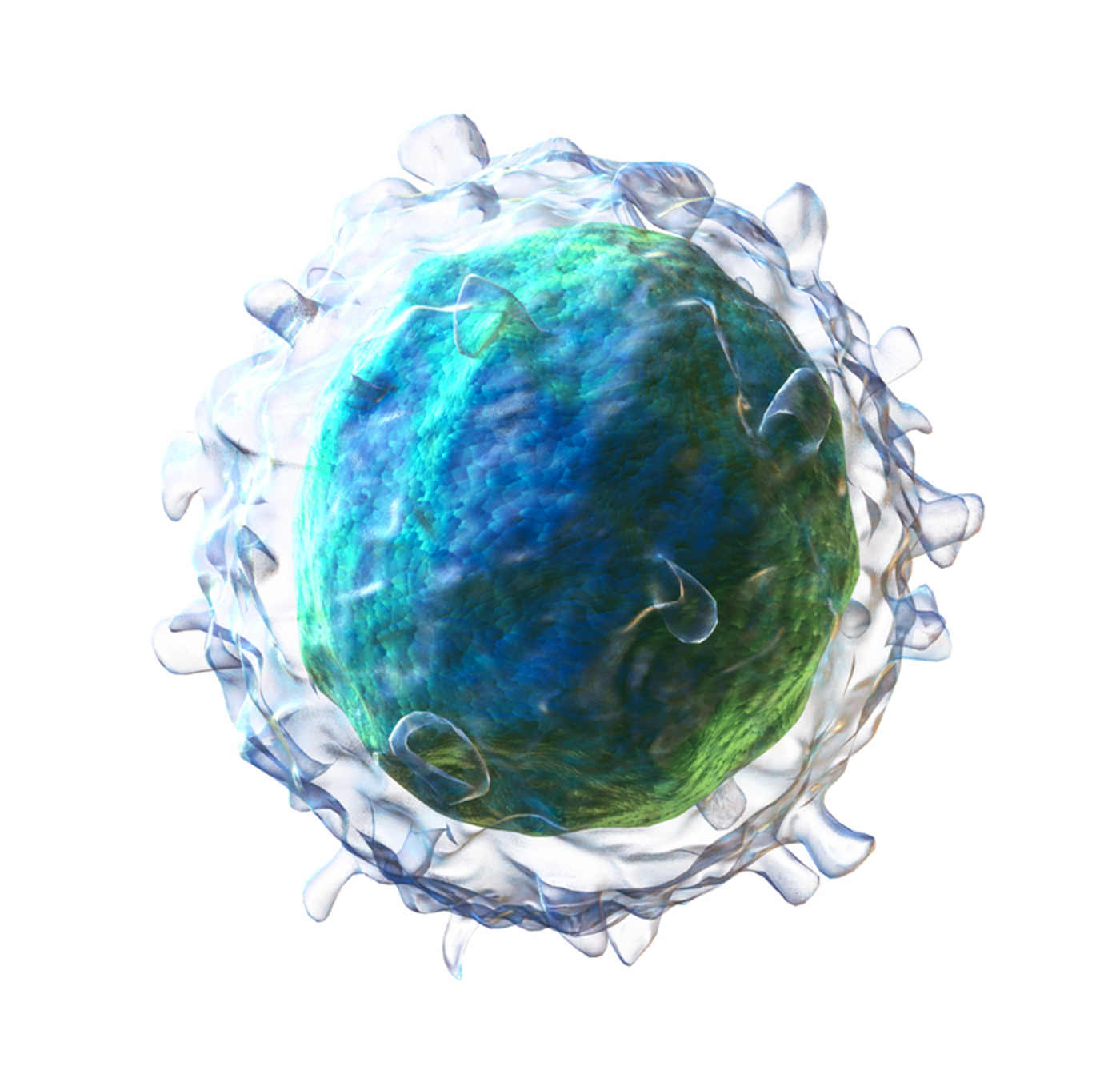
The two broad networks are those in the gastrointestinal tract and in blood-rich regions: blood, bone marrow, spleen, and lungs. B cells, which produce antibodies, are undeniably vital for immunity and for a healthy microbiome. In response to a pathogenic invasion, B cells go through clonal expansion, where one “parent” cell “gives birth” to an army of identical clones. How the clones move into different tissues and disperse themselves greatly impacts how well the body controls infections.
After analysis, researchers saw that there are more memory B cells in the gut network than in the blood-rich network, and the B cell populations are more related genetically.
"Presumably, this is because the gut is one of the organs that is constantly bombarded by stimuli from the environment, whether the stimuli that drive these B-cell clones are derived from the microbiome or other pathogens is not yet known," Prak said.
The development of the sequencing technology that resulted in the discovery of the “B cell clonal geography” took researchers over two years. Using the technology, they traced more than 933,000 B cell lineages after sequencing the heavy-chain variable domain region of the B cell gene. This particular region is largely responsible for the diversity of antibodies that helps the body defend against and remember a vast amount of pathogens.
“Our fantasy for the future is to create organ-specific immune monitoring assays,” Prak said. “If we can define features of the antibody repertoire that are unique to particular tissues, we may be able to monitor tissue-specific immune responses using blood-based clinical lab tests."
Prak’s plans would be extremely valuable for the monitoring of autoimmune diseases and the different responses individuals have to vaccines.
The present study was published in the journal Nature Biotechnology.