Researchers at the University of Alabama at Birmingham want to know how
E. coli untangles proteins when they become aggregated. They reason that their findings can be applied to ailments such as Alzheimer’s, Parkinson’s and
prion diseases, all of which involve “tangled” proteins. Misfolded beta-amyloid and tau proteins accumulate in the brain during Alzheimer’s disease, and misfolded alpha-synuclein has been implicated in Parkinson’s disease. Prions are a type of “infectious” protein responsible for neurodegenerative conditions such as
Creutzfeld-Jacob disease. These protein aggregates are thought to disrupt the normal function of cells, causing various disease symptoms.
The team, led by Aaron Lucius, professor of Chemistry, discovered that the
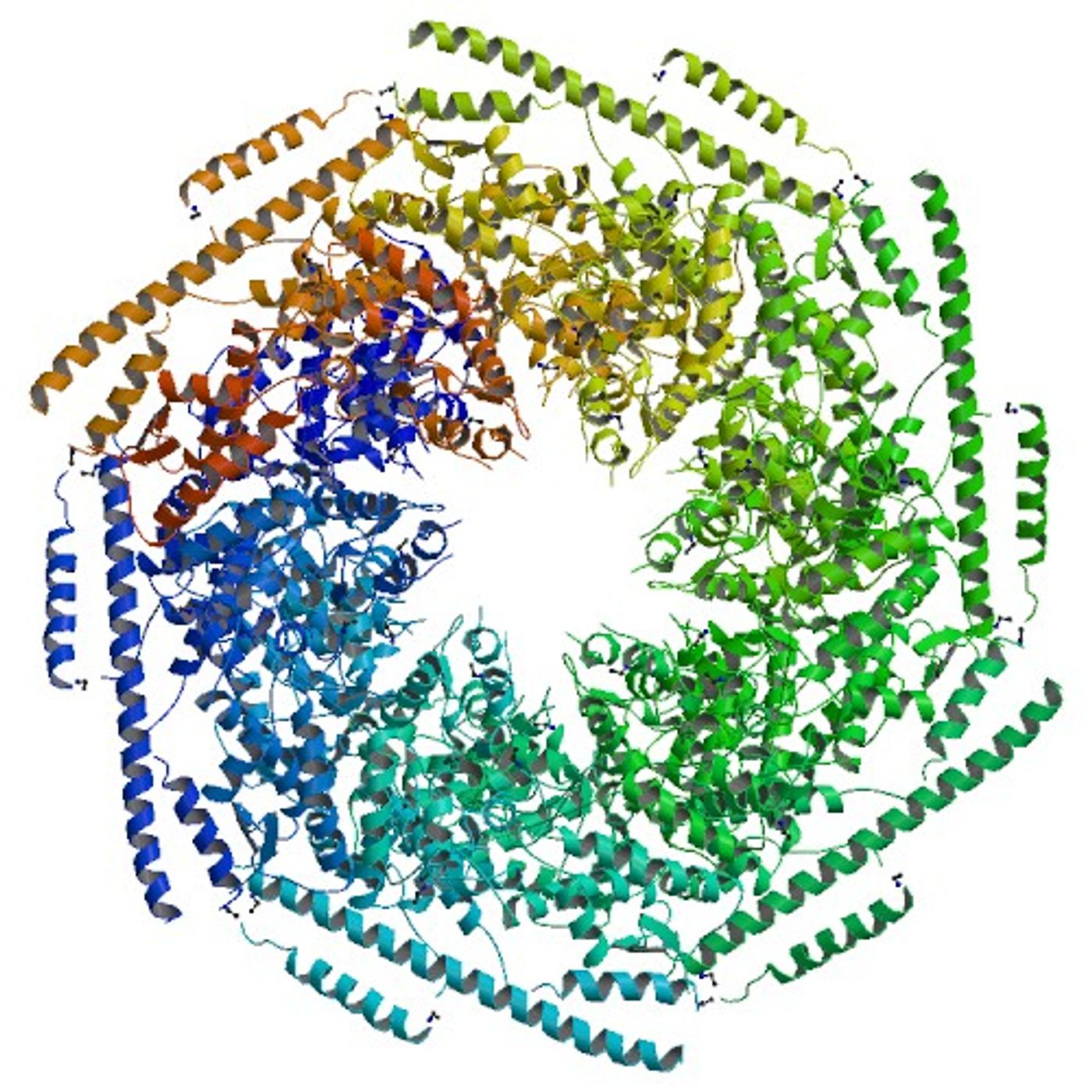
E. coli protein ClpB (caseinolytic protease B) uses a novel mechanism to untangle protein aggregates. ClpB is an
ATPase and belongs to the
hexameric AAA+ superfamily of enzymes. It is produced when cells experience stressful conditions, such as high heat, that denature and aggregate proteins. The ClpB enzyme consists of six subunits that form a hexagon with a channel in the middle.
ClpB was long thought to function like its counterpart ClpA. ClpA feeds misfolded proteins through its central channel into the protease ClpP, where they are degraded. ClpA does this by a mechanism of “processive pulling”, continually feeding long strands of protein into ClpP. What Lucius and his team found, however, was surprising. "Oour results support a molecular mechanism where ClpB catalyzes protein disaggregation by tugging and releasing exposed tails or loops”, says Lucius.
They reason that such a “tugging” mechanism could be used by other enzymes, and may inform therapeutics for Alzheimer’s or Parkinson’s disease. According to Lucius, “we don’t know how proteins get tangled, but if we can study how proteins get disaggregated, it may have clinical relevance”.
Sources:
Phys.org,
Biochemical Journal, Wikipedia