The First Treatment for Ebola is Approved by FDA
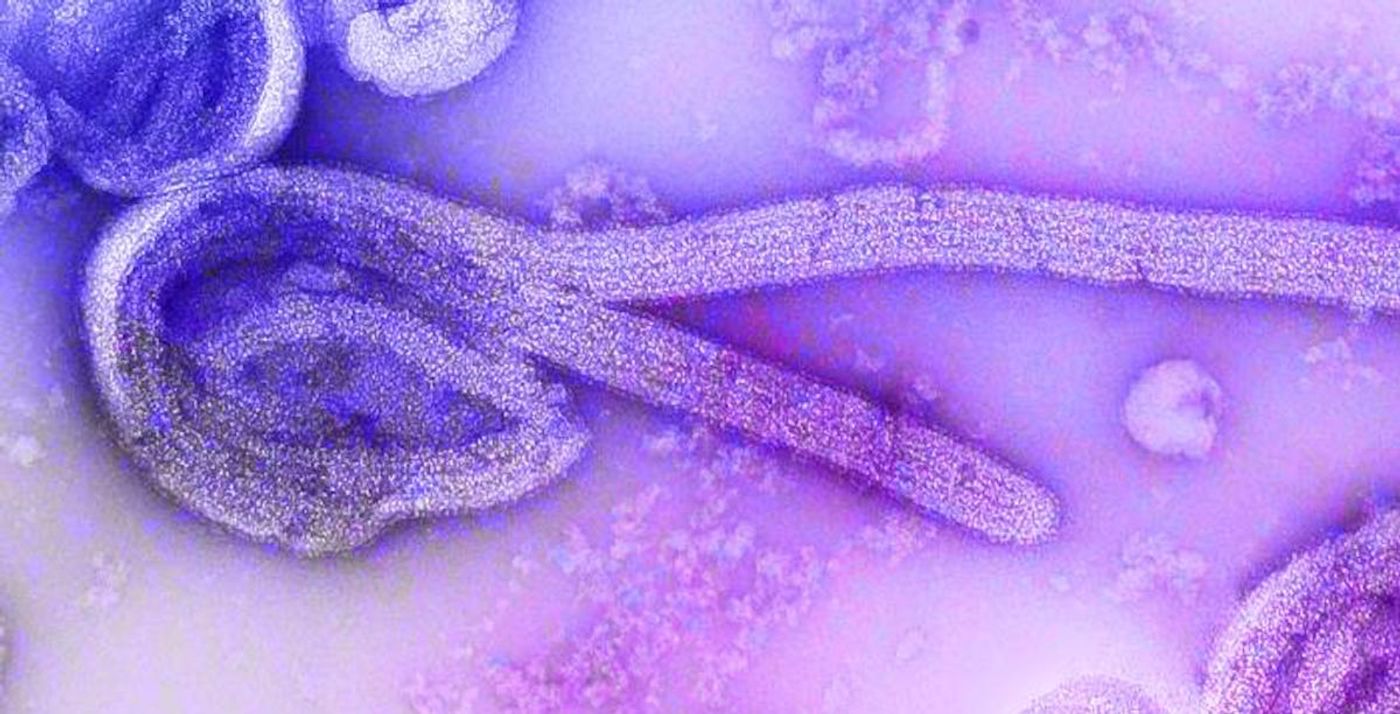
Ebola virus can pass from animals to humans, and between people through direct contact with infected fluids or tissues. Rarely, it causes outbreaks but when it does, they can lead to severe illness, hemorrhagic fever, and death. Symptoms are often similar to some common disorders; in an early phase an infected person might experience fever, muscle pain, and headache, for example, and later symptoms include vomiting, diarrhea, and rash. Fatality rates have ranged from 25 to 90 percent in various outbreaks. Supportive measures like rehydration can improve survival, but for a long time, there was no treatment.
That's changed; the Food and Drug Administration has now approved a therapeutic for the Zaire ebolavirus (Ebola virus) strain, one of the four that can cause disease in humans. The new drug, Inmazeb, is a combination of monoclonal antibodies: atoltivimab, maftivimab, and odesivimab-ebgn. The results from the clinical trial show that it can reduce mortality from about 50 to 30 percent.
The surface of the Ebola virus carries a glycoprotein that is the target of Inmazeb. During an Ebola infection, the glycoprotein attaches to a receptor on host cells, fusing the membranes of the viral and host cells, and giving the virus entry. The three Inmazeb antibodies can neutralize the glycoprotein; they bind to it and stop it from attaching to the host cell and entering it.
The drug was tested in 382 Ebola virus patients of various ages during the PALM clinical trial and during the 2018-2019 Ebola virus outbreak in the Democratic Republic of the Congo (DRC). In the PALM trial, participants were given either Inmazeb or a control. In the group of 154 people that got Inmazeb, about a third (33.8 percent) had died after 28 days of infection, while in the group of 153 that got the control, about half (51 percent) had died by 28 days.
“Today’s approval highlights the importance of international collaboration in the fight against Ebola virus,” said John Farley, M.D., MPH, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research. “The urgent need for advanced therapies to combat this infectious disease is clear, and today’s action is a significant step forward in that effort.”
The drug has been developed by Regeneron, and should not be used while a person has gotten a vaccine for the Ebola virus, because it can isrupt the vaccine's efficay. The vaccine, which was approved by the FDA in December 2019, is called Ervebo, and was tested during the 2014-2016 Ebola outbreak in Guinea.
New work by researchers at the University of Delaware has also now revealed more about the structure of the Ebola virus, which is made up of a viral genome inside of a nucleocapsid that consists of nucleoproteins that are arranged like a slinky. The study, which was reported in the Journal of Chemical Physics, found that the integrity of the nucleocapsid is dependent on the presence of the single-stranded RNA genome. Without it, the nucleocapsid begins to deform.
"As basic scientists we are excited to understand the fundamental principles of Ebola," said research leader Professor Juan Perilla. "The nucleocapsid is the most abundant protein in the virus and it's highly immunogenic -- able to produce an immune response. Thus, our new findings may facilitate the development of new antiviral treatments."
Sources: FDA, AAAS/Eurekalert! via University of Delaware, Journal of Chemical Physics