The Molecular Gate That Enables SARS-CoV-2 to Infect Cells
Researchers investigating how the SARS-CoV-2 virus gets into cells to cause COVID-19 learned early on in the pandemic that the spike protein of the virus binds to receptors called ACE2, which can be found on human cells in the nose. Scientists have also been learning more about the viral spike protein, like how it is structured, and what sugar residues, which are in a chemical group known as glycans, stick to the spike.
Glycans have a variety of biological roles, but scientists have now found that when it comes to SARS-CoV-2, they act like 'gates' on the spike protein that open up and give SARS-CoV-2 entry into cells. This work has been reported in Nature Chemistry.
“We essentially figured out how the spike actually opens and infects,” said senior study author Rommie Amaro, a professor of chemistry and biochemistry at the University of California San Diego. “We’ve unlocked an important secret of the spike in how it infects cells. Without this gate the virus basically is rendered incapable of infection.”
Other researchers have been trying to find ways to use these glycan residues to prevent COVID-19. Amara also suggested that this work can help scientists take advantage of the glycans in a therapeutic approach that might help prevent COVID-19; if the glycan gate can be 'locked,' it could potentially prevent infection.
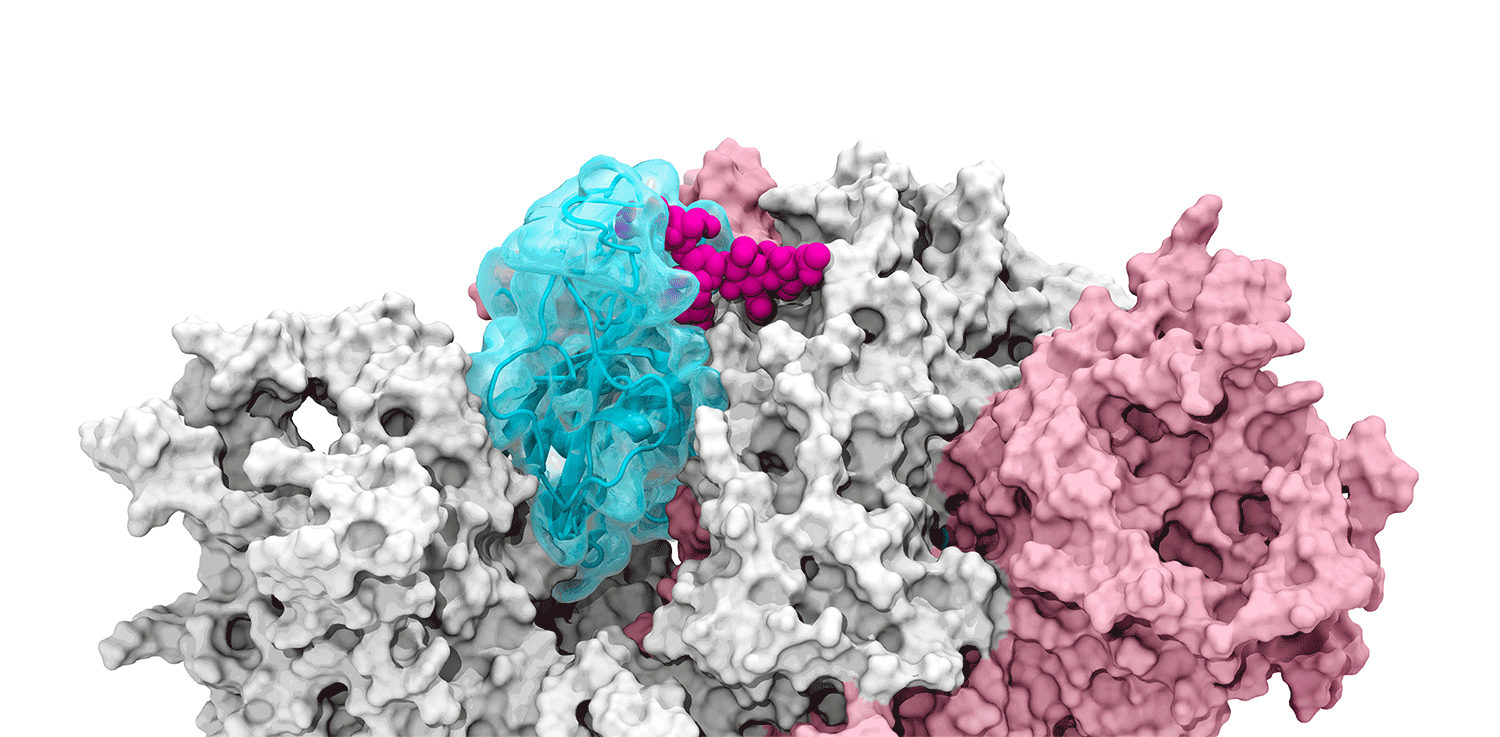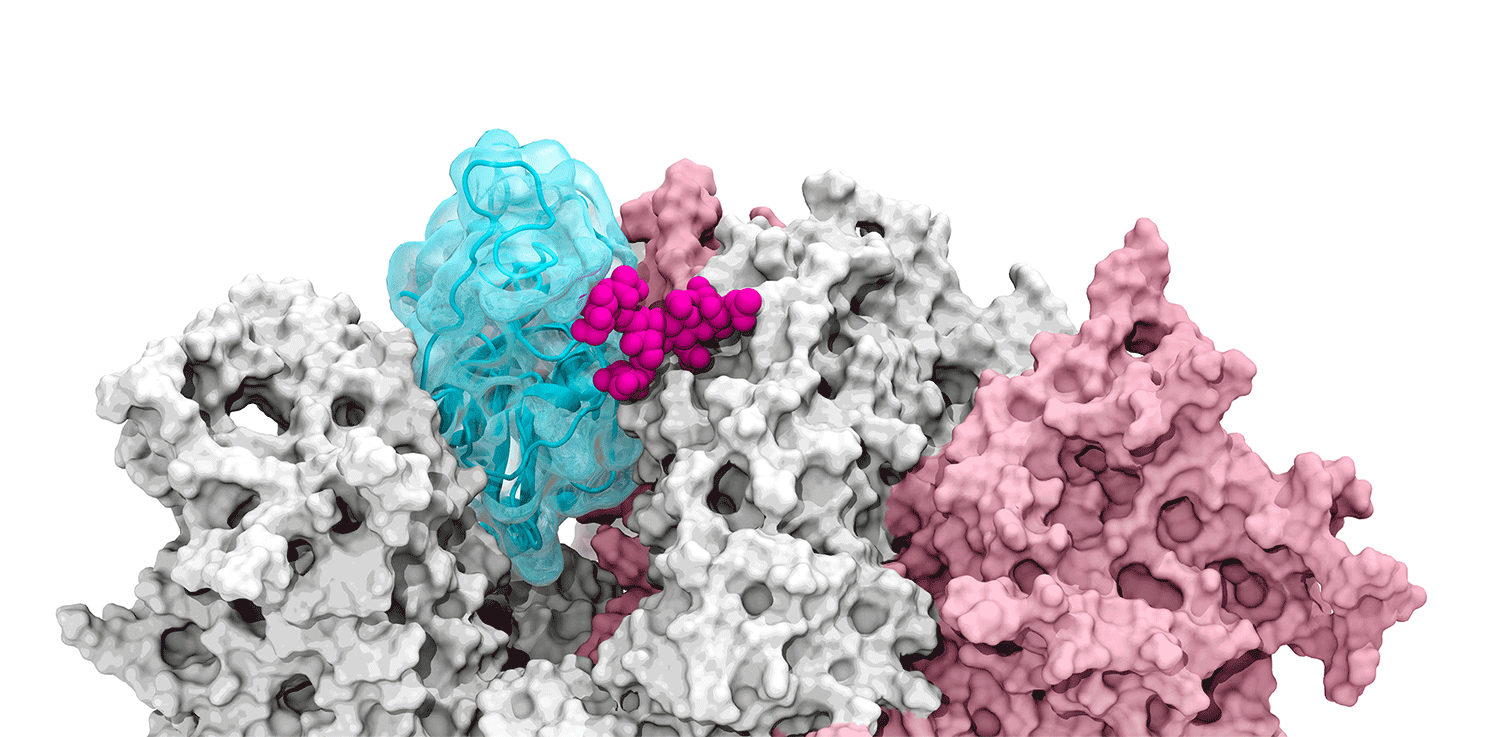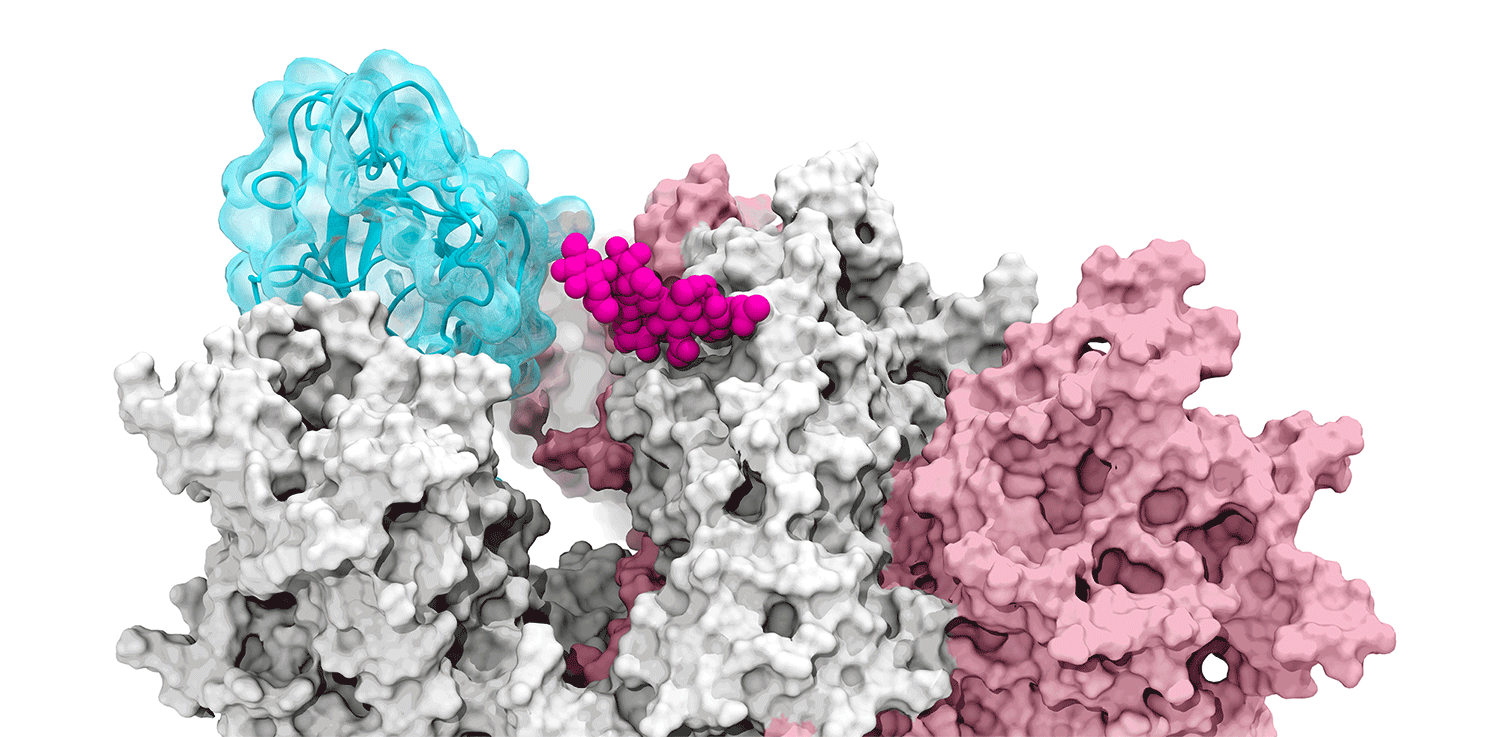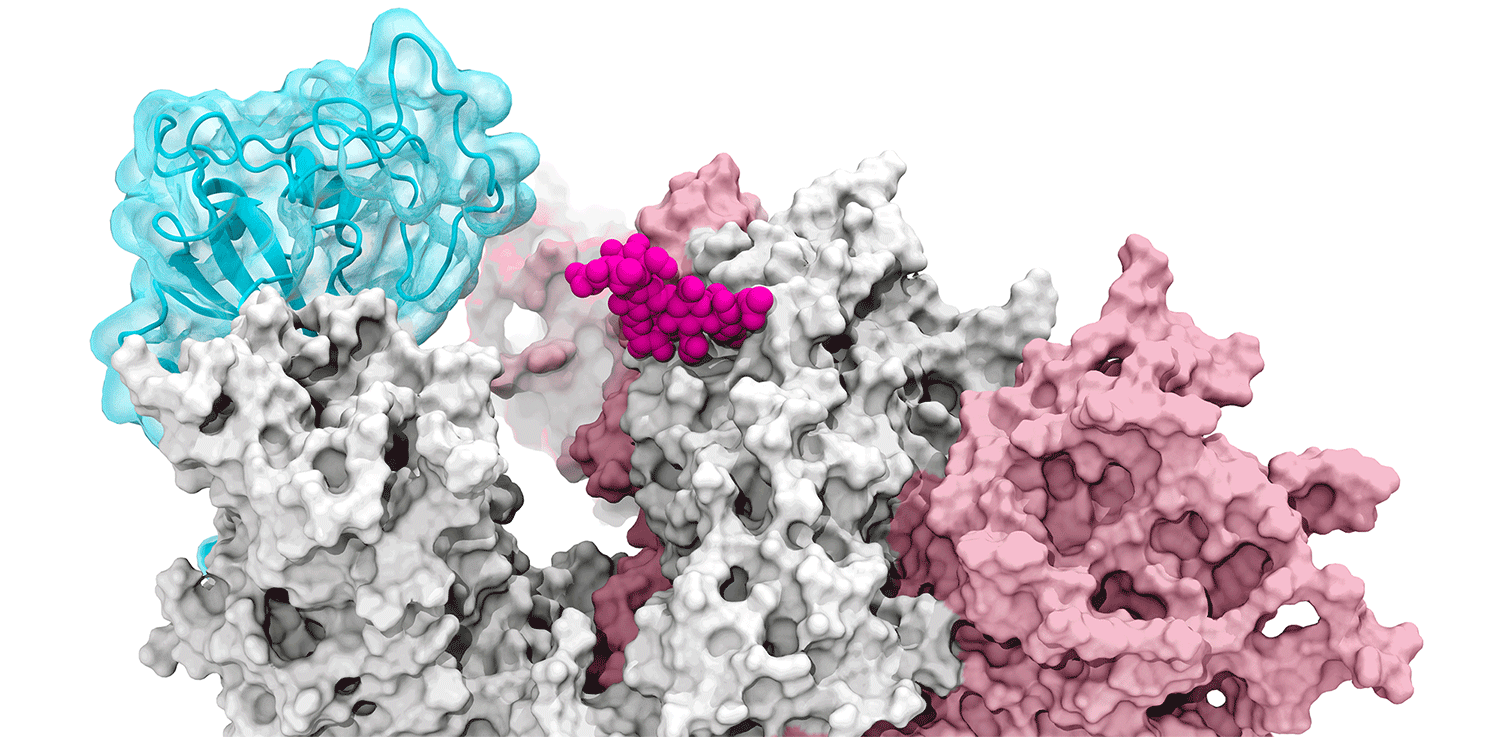
The glycans coating the the spike protein seem to shield it from the immune system, which may see the sugars as harmless. Previous research on the glycans has shown the spike protein in one position. This research used supercomputers to simulate the dynamic movement of the glycans on the spike protein, illuminating a new aspect of the mechanism of infection.
“We were actually able to watch the opening and closing. That’s one of the really cool things these simulations give you: the ability to see really detailed movies. When you watch them you realize you’re seeing something that we otherwise would have ignored,” said Amaro. "It’s only because we captured the movie of the whole process that you actually see it doing its thing.”
The supercomputers at UC San Diego and the Texas Advanced Computing Center (TACC) at the University of Texas (UT) Austin enabled the researchers to look at 300 different perspectives of the portion of the spike protein that attaches to the ACE2 receptor - the receptor binding domain (RBD). One glycan, called N343, pried the RBD into position to let it link to ACE2. The scientists compared this movement to a “molecular crowbar."
Without the glycan gate, the RBD cannot move into a conformation that will trigger the infection, noted Jason McLellan, an associate professor of molecular biosciences at UT Austin.
Sources: UC San Diego, Nature Chemistry