The bacterium Mycobacterium tuberculosis is among the world’s deadliest infectious pathogens. Those bacteria are able to utilize two alternate forms of metabolism depending on the needs of the microbe, and can switch back and forth between the two.
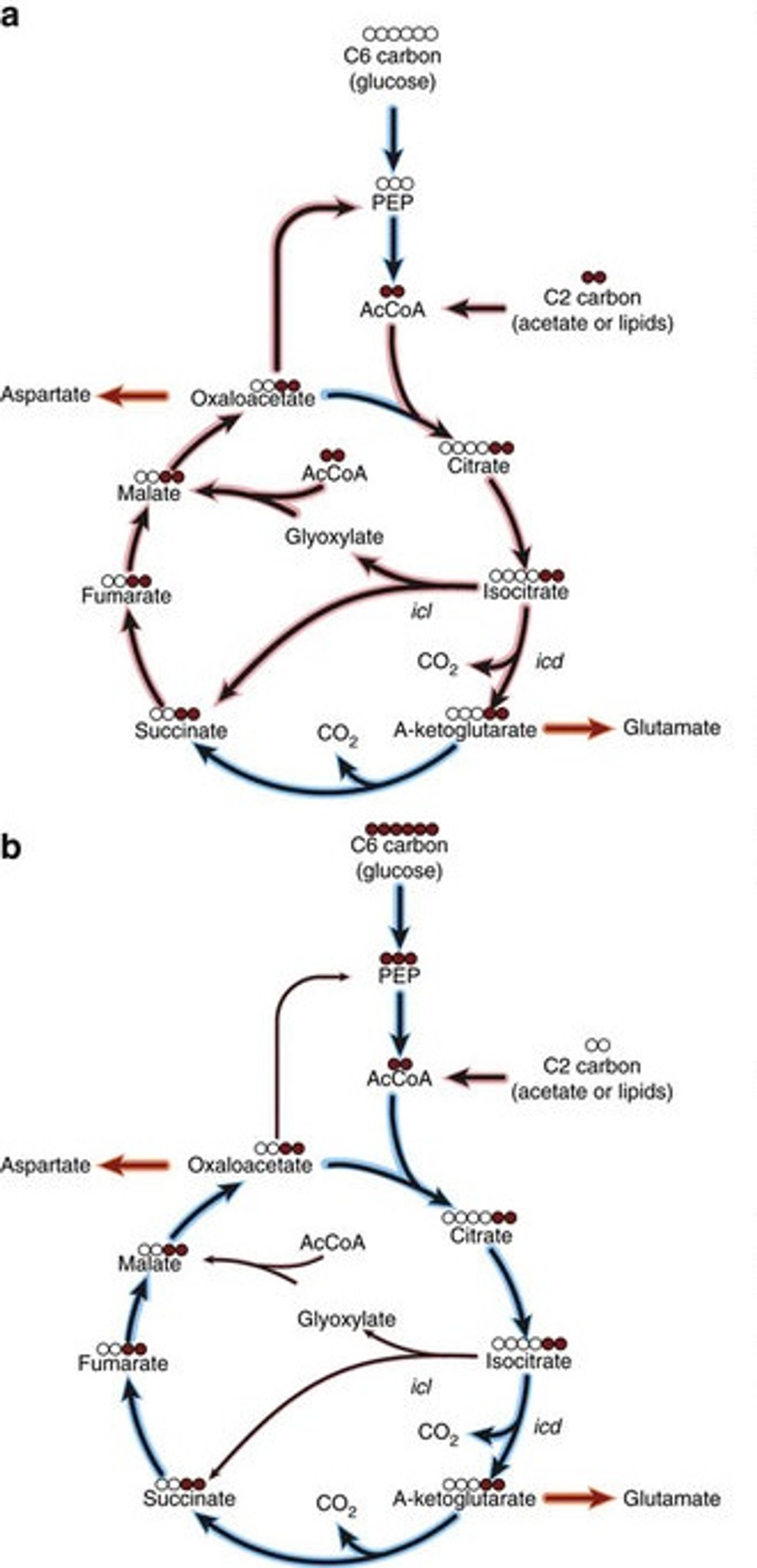
In the course of an infection with M. tuberculosis, the bacteria needs energy, which it can get from the breakdown of lipids and fatty acids. In the course of the chemical reactions that cycle through the metabolism of those materials, a molecule is produced that is used for a different chemical reaction the microbe requires for synthesis of parts. The bacterium is able to optimize its chances for survival by maintaining the ideal balance between the energy producing reaction, or the biosynthesis reaction.
The molecule used in the different reactions is isocitrate. It seems as though the path the chemical takes is under the influence of enzymes that are key to the various chemical reactions in question.
Isocitrate dehydrogenase is an enzyme that maintains isocitrate as part of the fat metablism pathways; isocitrate lyase and maltase synthase move isocitrate into the biosynthetic pathway. While it is being used for biosynthesis, energy production is halted unless isocitrate is in continuous supply. Loss of the molecule is devastating to the bacterium, which means it could be a great way to kill bacterial infections from the pathogen.
Researchers at Swiss Federal Institute of Technology in Lausanne (EPFL) in the lab of John McKinney worked in collaboration with the lab of Uwe Sauer at Swiss Federal Institute of Technology in Zürich (ETH Zürich) to investigate how mycobacteria control the enzymes in charge of the balance of the reactions. To do so, they simply deleted the genes responsible for the enzymes in various strains of mycobacteria.
Their
results, which were published in Nature Communications, demonstrated that there is no simple on-off switch behind the expression of the enzymes. The lead author of the work, Paul Murima, likens it to a thermostat. "If the temperature becomes too high, a thermostat cools the house down; if it gets too low it heats it up. Similarly, the mechanism that controls how isocitrate is used responds to negative feedback, and so it dampens 'noise' to maintain optimal levels,” he explained.
This makes the microbe able to quickly and easily respond to changes in the environment that affect what might be available as energy sources. Other pathogenic microbes like leprosy are mycobacteria that use this same survival strategy. While the mechanism is similar to that used by gut bacteria like E.coli, it is also different enough to mean that treatments that target mycobacteria would not affect the microbiome, making it a very attractive candidate for drug targets.
Sources:
AAAS/Eurekalert! via
EPFL,
Nature Communications