Bezlotoxumab for recurrent C. difficile infections
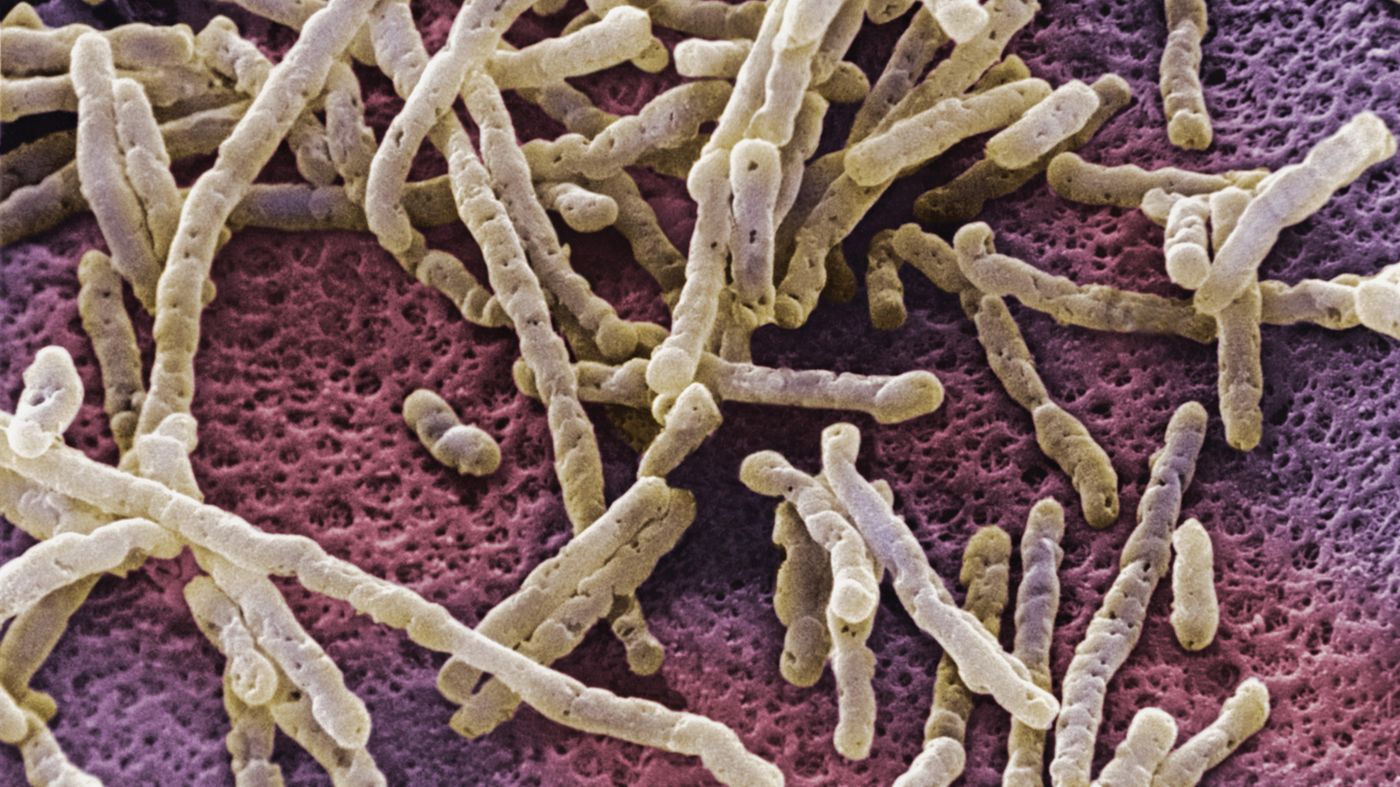
Clostridium difficile often infects hospitalized patients who have undergone antibiotic therapy. These bacteria infect the gut, causing diarrhea and colitis. These infections are serious and can even be fatal.
Although C. difficile can be treated with antibiotics, the infection often recurs.
According to study author Mark Wilcox from the University of Leeds, “about one in four patients who have been treated with antibiotics for an initial C.diff infection will go on to have a repeat infection. These repeat infections are more difficult to treat, have more severe outcomes for the patient, and are associated with more hospitalisations. It is important to treat the first episodes of C. diff infection optimally, as each recurrence increases the chance of another episode even more.”
With this in mind, Wilcox and colleagues conducted two phase 3 trials to determine if monoclonal antibodies against C. difficile toxins could help prevent recurrent infections. Bezlotoxumab is a monoclonal antibody against toxin B, and actoxumab is a monoclonal antibody for toxin A.
The studies (MODIFY I and MODIFY II) were conducted with 2,655 adults at 322 sites in 30 countries. Participants received, in addition to standard antibiotic treatment (vancomycin, metronidazole, or fidaxomicin), bezlotoxumab, actoxumab, both bezlotoxumab and actoxumab, or a placebo (saline).
In the MODIFY I study, infection recurred (within 12 weeks of initial cure) in 28% of participants receiving the placebo, but only in 17% of people receiving bezlotoxumab. The rates were very similar in the MODIFY II study. The effect of actoxumab and bezlotoxumab together was similar to that of bezlotoxumab alone. In addition, the rates of recurrent infection in high-risk patients were lower for the bezlotoxumab group and the combined actoxumab/bezlotoxumab groups compared to the placebo group.
The researchers also looked at an endpoint they termed “sustained cure,” defined as initial cure with no recurrent infection over the next 12 weeks. In pooled data from the MODIFY I and II studies, 64% of participants in the bezlotoxumab group achieved sustained cure, compared to 58% in the actoxumab/bezlotoxumab group and 54% in the placebo group.
Overall, bezlotoxumab lowered the risk of first post-treatment relapse by 40% compared to standard treatment alone.
Bezlotoxumab is approved by the FDA and will soon be available to doctors treating C. difficile infections. However, in a commentary on this study, John G. Bartlett questions whether using bezlotoxumab is really cost-effective. He notes that, while effective, bezlotoxumab may not need to become the standard of care - fidaxomicin already effectively prevents recurrent infections, and antibiotic resistance has not been an issue for treating C. difficile infections.
This new treatment regimen may, however, benefit high-risk patients - those older than 65, people with kidney issues, or those already on antibiotics, for example. "The studies showed that bezlotoxumab was particularly effective in those patients with risk factors for poor outcome, including older age, immunocompromise, and severe infection," says Wilcox.
Sources: NEJM article, NEJM commentary, Science Daily