Relieving a Mechanism That Could Underlie as Many as 20 Rare Disorders
Proteins are critical to organisms; they carry out necessary functions and form essential structures. Coding sequences in the genome are used by cells to create strings of amino acids, which have to be properly folded into a three-dimensional structure to form proteins. Problems with protein structure can lead to various physiological problems and disease.
Toxic proteinopathies are a group of inherited diseases that are caused when improperly folded proteins accumulate in cells. Now researchers have learned more about a mechanism that may be causing several of these disorders - a dysfunction in a process called the secretory pathway, which sends proteins for disposal, or to the cell surface. Scientists from the Broad Institute of MIT and Harvard and Brigham and Women's Hospital (BWH) have reported these findings in Cell.
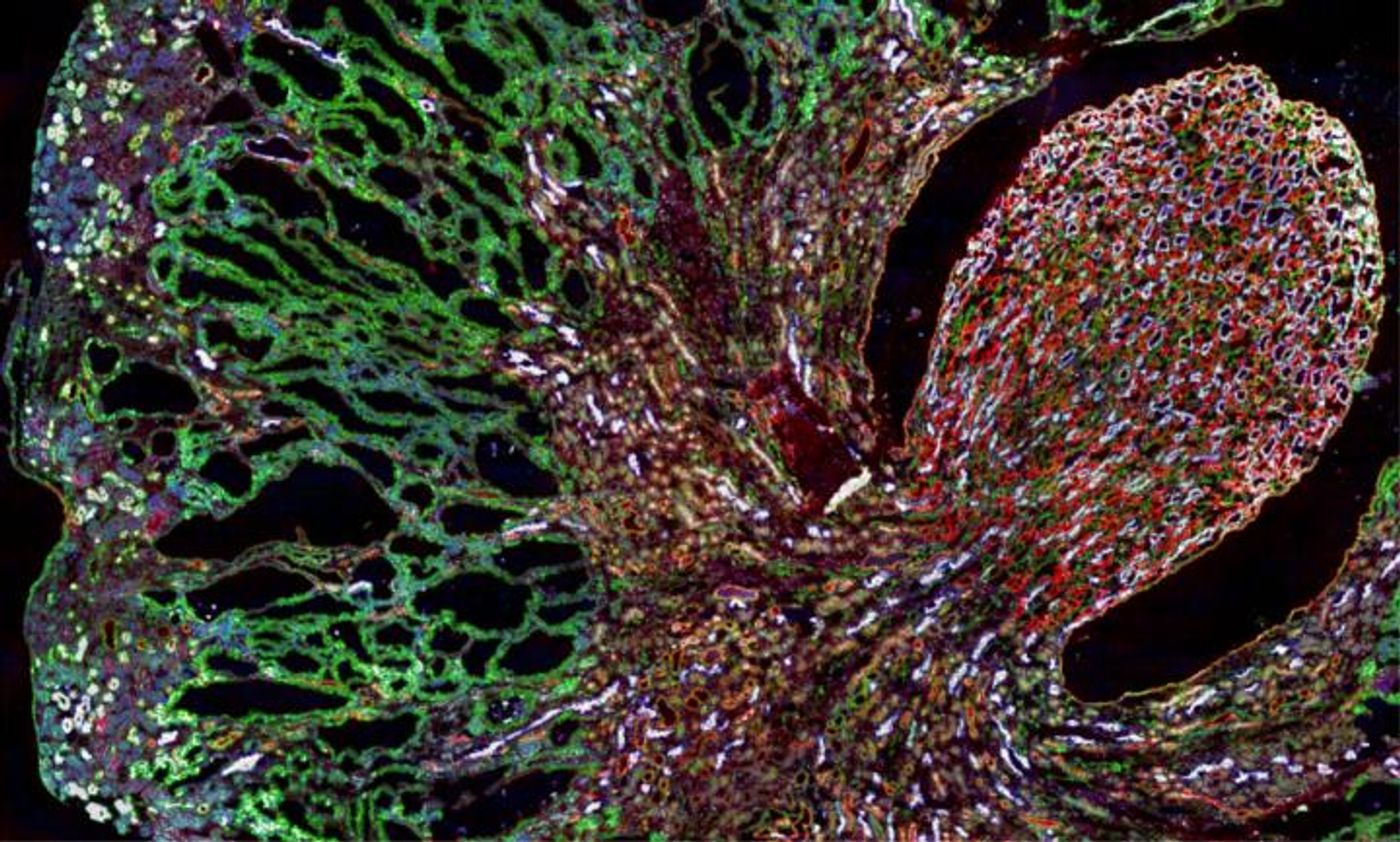
The researchers were investigating MUC1 kidney disease (MKD), a rare kidney disorder caused by a mutation in the MUC1 gene, which creates a misfolded MUC1 protein that accumulates in kidney cells. The cell doesn’t eliminate the aberrant protein because of a sort of secretory pathway traffic jam. A compound, BRD4780, could alleviate the problem.
"What blew my mind is that, in the process of studying the mechanism behind MKD, we have uncovered fascinating new biology about how cells handle misfolded proteins, and these key insights may help us address several devastating diseases," noted study senior author Anna Greka, director of the Broad's Kidney Disease Initiative (KDI), who also has appointments at Harvard Medical School and BWH. "Our team is working around the clock to translate these discoveries into a new therapy we can bring to patients as quickly as possible."
The compound rescued the pathway defect in a variety of models, including kidney organoids (mini-kidney models), human kidney cells, and an animal MKD model. Researchers can now use BRD4780 to development treatments for MKD and other toxic proteinopathies. There are no therapies available for these disorders right now.
The scientists also found how it was working; BRD4780 binds to a receptor called TMED9, not the misfolded protein. In the MKD model, the TMED9 receptor was found to be trapping the aberrant protein, which prevented it from moving to the lysosome, where it would normally be degraded. The improperly folded protein was then accumulating and causing problems for kidney cells, which eventually die because of it. When BRD4780 attached to TMED9, however, the receptor released the protein, and the cell could then degrade it.
When the team blocked TMED9 expression using CRISPR, they saw the same result.
"This is completely new biology," Greka said. "We did not know that a cargo receptor like TMED9 could block and ultimately interfere with the destruction of a misfolded protein. And the question became, is the same biology at work in other conditions caused by a buildup of misfolded proteins?"
Over fifty diseases are toxic proteinopathies. The researchers suggested that BRD4780 might be of therapeutic use for twenty diseases in which misfolded proteins get trapped.
"Many of these disorders may tie back to the same mechanism," Greka explained. "Our next step is to develop a deeper understanding of cargo receptors, why they stop misfolded proteins from being eliminated as they should and figure out exactly how to develop drugs to counter them."
Sources: AAAS/Eurekalert! Via Broad Institute, Cell