Treating Huntington's Disease With a Gene Therapy That Targets Brain Cells
Huntington's disease (HD) is a rare degenerative brain disorder that is caused by mutations in the Huntington gene (Htt), and neurodegeneration in a part of the brain called the striatum. The most common type appears in a patient's thirties or forties, symptoms include emotional problems, cognitive dysfunction, and uncontrolled twitching or jerking, called chorea. These movements get worse as the disease progresses. People with Huntington's disease might eventually have difficulty swallowing or walking, and their ability to think and reason declines. They live around twenty years after the onset of symptoms.
Researchers may have now found a way to treat the disorder by stimulating the growth of new neurons. The work, led by Dr. Gong Chen of Jinan University in China, formerly a professor at Penn State University, has been published in Nature Communications.
"We are developing a series of NeuroD1-based gene therapies to reprogram brain internal glial cells directly into functional new neurons to treat a variety of brain disorders including Huntington's Disease, Alzheimer's disease, stroke, ALS, and many more," said Dr. Chen. "Because every single neuron in our brain is surrounded by supporting glial cells, such direct glia-to-neuron conversion technology offers great advantages over stem cell transplantation therapy in terms of high efficiency of neuroregeneration and no worries about immunorejection," Dr. Chen added.
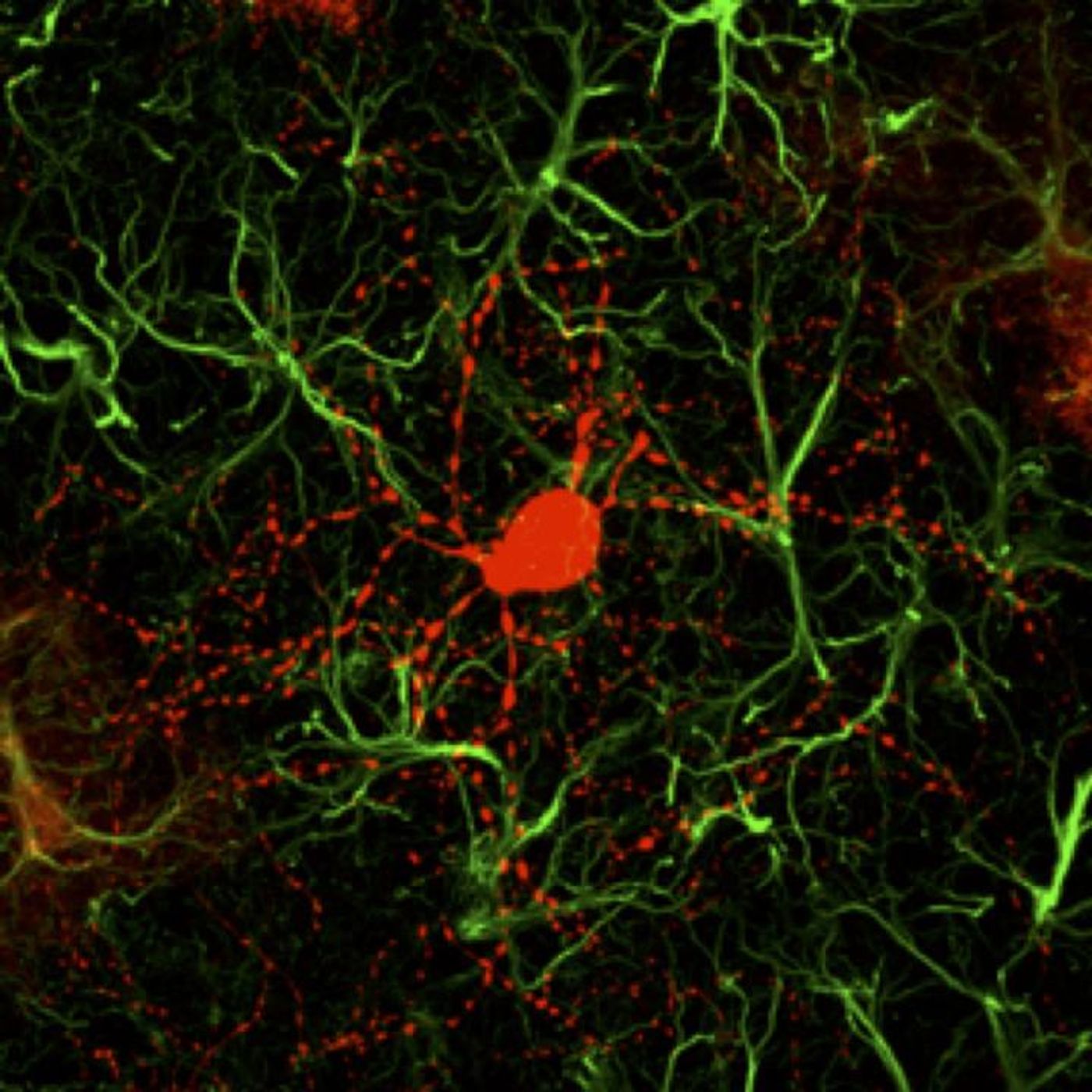
NeuroD1 is a transcription factor, meaning it helps control the activity of other genes. Previous work by Chen's team has shown that when NeuroD1 is expressed in the mouse brain, a type of brain cell called a cortical astrocyte will convert into a functioning neuron. The researchers then demonstrated that the findings hold true in non-human primate brains.
The neurons that are created using NeuroD1 as a trigger tend to be a kind of cell called a glutaminergic neuron. Around 80 percent of the brain is made of this type of neuron, and they are the primary source of brain activity. Huntington's disease, however, is caused by the degeneration of a type of cell called a GABAergic neuron; these inhibitory neurons are responsible for about 90 percent of the activity in the striatum. The scientists found a way to stimulate the creation of these neurons.
"In order to generate GABAergic neurons, we combined NeuroD1 together with another transcription factor Dlx2, which is known to generate GABAergic neurons during early brain development, and successfully converted striatal astrocytes into GABAergic neurons in HD mice," said the first author of the study Dr. Zheng Wu. "Importantly, here we used the adeno-associated virus (AAV) vectors, which have been approved by FDA as a common gene therapy vector in many clinical trials, to develop a novel gene therapy for the treatment of HD."
Striatal astrocytes were converted directly into GABAergic neurons in about 80 percent of AAV-infected cells, and the astrocytes that remained were able to proliferate so their levels would return to normal. The newly-made neurons were functional and formed connections with other neurons as well as pushing their axons into the correct places.
"The most exciting findings of this HD study are the significant motor functional recovery and remarkable extension of life span among the gene therapy-treated HD mice," Chen noted.
"Our regenerative gene therapy approach is different from conventional gene therapy that typically aims at the mutant genes by either correcting the gene mutations or reducing the mutant gene product, such as reducing mHtt aggregates in HD patients," added Chen. "Obviously, reducing mHtt aggregates at early stage might slow down the disease progression but it cannot regenerate new neurons for the late-stage patients. An ideal approach may be to combine our neuroregenerative approach together with gene correction technology to generate healthy new neurons in future studies."
Sources: AAAS/Eurekalert! via Guangdong-Hongkong-Macau Institute of CNS Regeneration - Jinan University, Nature Communications