Human Cells Modify COVID-19's Spike Protein, Making it Flex
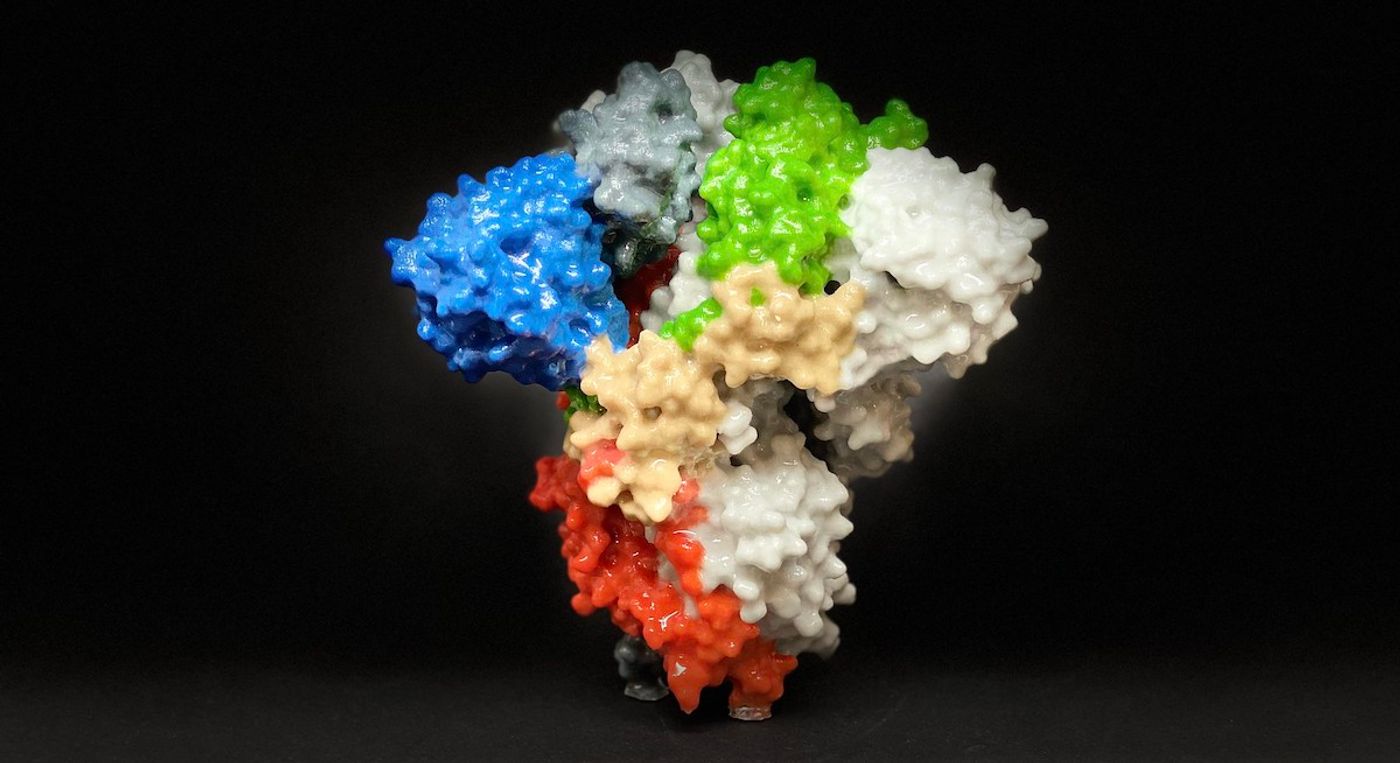
SARS-CoV-2 uses a spike protein to bind to human cells and infect them. Researchers have now found that human cells can modify that spike protein, changing it in ways that make it more flexible. This may also make the spike protein better at infecting other cells and evading antibodies as well. The findings have been reported in the Proceedings of the National Academy of Sciences.
In this work, researchers generated a computational model of the spike protein with atomic-level details. They were able to run simulations with that model, which revealed how human cells can change the spike protein. This study is the first to reveal these details about the SARS-CoV-2 spike protein, said the researchers.
The study authors noted that while we know a lot about the variants that have arisen, less is known about how the SARS-CoV-2 virus is modified by human cells after an infection starts. Human cells can naturally make many types of post-translational modifications, such as glycosylation, the addition of glycans that are a type of sugar molecule. This research showed that there are "significant dynamic differences" between spike proteins that have been glycosylated or not, noted graduate student Tianle Chen.
The dynamics of the spike protein, like how it flexes and moves as it searches for a binding partner, are crucial, said Emad Tajkhorshid, a biochemistry professor at the University of Illinois Urbana-Champaign.
The glycans on the spike protein were found to interact with human cells, which allowed the spike to search for a receptor on the membrane. The spike protein was given a wider range of motion.
The researchers identified several moving parts on the SARS-CoV-2 spike protein that act like hinges. Thus, the spike protein can stick out and swivel around. It can also shift from one structure to another, through inactive and active forms. The scientists mapped the conformations revealed by the computational simulations. The study authors noted that the types of structures they saw are similar to what has been observed experimentally, which lends support to the work.
"In order to have a realistic representation, you have to look at the protein at the atomic level. We hope that the results of our simulations can be used for developing new treatments," said Tajkhorshid.
Glycosylation can act to shield the spike protein from the immune system as well as increasing its chances of infecting cells, noted postdoctoral researcher Karan Kapoor. "Thus, the functions of these post-translational modifications are much wider than what was initially thought. This understanding can now provide additional opportunities for targeting the function of this virus," Kapoor said.
"The hope is that down the road, this new understanding of the spike protein is going to be useful for therapeutic efforts. I imagine we can target the dynamics of the spike protein with compounds that bind to the hinges and make them inflexible, and therefore in principle, make the virus less effective," Tajkhorshid said.
Sources: University of Illinois Champagne, PNAS